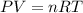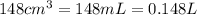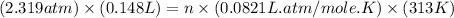Answers: 1
Another question on Chemistry

Chemistry, 21.06.2019 21:00
Solar energy is energy from the sun that is converted into thermal or energy. a. nuclear b. mechanical c. electrical d. chemical
Answers: 2

Chemistry, 22.06.2019 00:30
Which compounds have the empirical formula ch2o? a.c2h4o2 b.c3h6o3 c.ch2o2 d.c5h10o5 e.c6h12o6
Answers: 3

Chemistry, 22.06.2019 14:50
Which of the following is most likely true about water in chemical systems? a) water dissolves nonpolar ionic compounds. b) water dissociates ionic compounds. c) water dissociates covalent molecules. d) water dissolves nonpolar covalent substances.
Answers: 1

Chemistry, 22.06.2019 17:30
Energy defines the different "states" of matter. in no more than 3 sentences, describe the amount of kinetic energy that each of the 3 states of matter possesses and relate that to the atom/molecular motion of each "state".
Answers: 2
You know the right answer?
At 40.0°c, the pressure inside a nitrogen-filled tennis ball with a volume of 148 cm is 235 kpa. how...
Questions


History, 22.04.2020 00:43

Mathematics, 22.04.2020 00:43

Spanish, 22.04.2020 00:43

English, 22.04.2020 00:43


Social Studies, 22.04.2020 00:43






Mathematics, 22.04.2020 00:43




Mathematics, 22.04.2020 00:43

History, 22.04.2020 00:43


Social Studies, 22.04.2020 00:43