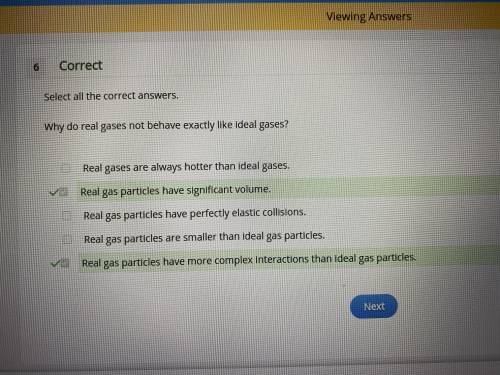
Select all the correct answers. why do real gases not behave exactly like ideal gases? real gases are always hotter than ideal gases. real gas particles have significant volume. real gas particles have perfectly elastic collisions. real gas particles are smaller than ideal gas particles. real gas particles have more complex interactions than ideal gas particles.

Answers: 2
Another question on Chemistry


Chemistry, 21.06.2019 22:30
Which type of bond is present in hydrogen sulfide (h2s)? the table of electronegativities is given. a. hydrogen b. ionic c. nonpolar covalent d. polar covalent
Answers: 1

Chemistry, 22.06.2019 07:00
At 450 mm hg a gas has a volume of 760 l, what is its volume at standard pressure
Answers: 2

Chemistry, 22.06.2019 16:00
Sulfuric acid is a polyprotic acid. write balanced chemical equations for the sequence of reactions that sulfuric acid can undergo when it's dissolved in water.
Answers: 2
You know the right answer?
Select all the correct answers. why do real gases not behave exactly like ideal gases? real gases a...
Questions




Social Studies, 24.02.2021 04:30

Biology, 24.02.2021 04:30





Mathematics, 24.02.2021 04:30




Biology, 24.02.2021 04:30

History, 24.02.2021 04:30


Mathematics, 24.02.2021 04:30

Social Studies, 24.02.2021 04:30


SAT, 24.02.2021 04:30