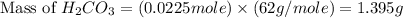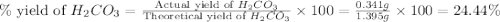Chemistry, 25.06.2019 17:10 sweetbri7p5v6tn
During a laboratory experiment, a 3.81-gram sample of nahco3 was thermally decomposed. in this experiment, carbon dioxide and water vapors escape and are combined to form carbonic acid. after decomposition, the sample weighed 2.86 grams. calculate the percentage yield of carbonic acid for the reaction. describe the calculation process in detail. nahco3 → na2co3 + h2co3

Answers: 1
Another question on Chemistry

Chemistry, 22.06.2019 07:30
What three things determine the shape and size of a puddle when water is poured out onto a surface
Answers: 2

Chemistry, 22.06.2019 22:00
Does the number of ions in solution increase, decrease, or remain constant? it continuously decreases. it continuously increases. it decreases at first, then increases. it increases at first, then decreases.
Answers: 3

Chemistry, 22.06.2019 22:30
Which of the following is not an assumption that scientists must make about the natural world? a. regularity b. causality c. predictability d. plausibility
Answers: 1

Chemistry, 23.06.2019 00:00
How many peaks will be present in a mass spectrum for brcl?
Answers: 1
You know the right answer?
During a laboratory experiment, a 3.81-gram sample of nahco3 was thermally decomposed. in this exper...
Questions


Mathematics, 13.07.2019 22:00

Social Studies, 13.07.2019 22:00







Advanced Placement (AP), 13.07.2019 22:00

Mathematics, 13.07.2019 22:00

Mathematics, 13.07.2019 22:00




Computers and Technology, 13.07.2019 22:00

Arts, 13.07.2019 22:00


Mathematics, 13.07.2019 22:00

Mathematics, 13.07.2019 22:00

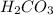 is, 24.44 %
is, 24.44 %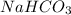 .
.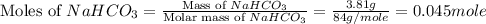
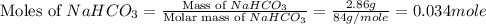
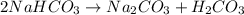
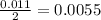 mole of
mole of 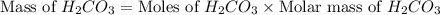
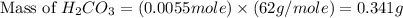
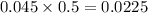 mole of
mole of