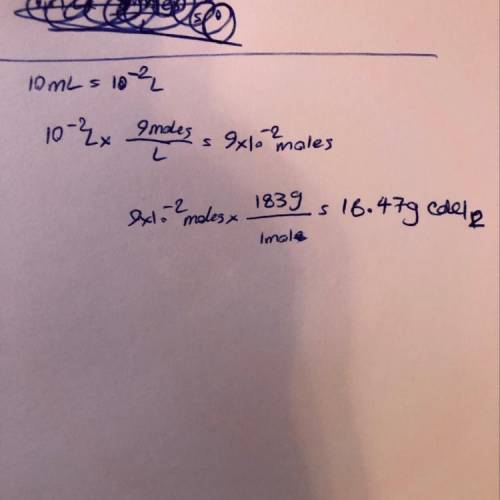Chemistry, 25.06.2019 01:30 daplol22222
The molar mass of cadmium chloride is 183 g. what mass of cdcl2 would be present in 10.0 ml of 9 m solution?

Answers: 1
Another question on Chemistry

Chemistry, 22.06.2019 09:30
In 2002, the rare earth elements mine in mountain pass, california was closed because
Answers: 1

Chemistry, 22.06.2019 13:50
Read the chemical equation. 2c2h2 + 5o2 → 4co2 + 2h2o which of the following statements would be correct if one mole of c2h2 was used in this reaction? one mole of oxygen was used in this reaction. five moles of oxygen were used in this reaction. four moles of carbon dioxide were produced from this reaction. two moles of carbon dioxide were produced from this reaction.
Answers: 3

Chemistry, 23.06.2019 03:30
27 drag each label to the correct location on the image. a particular exosolar system has five planets in total: a, b, c, d, and e. the table lists the orbital periods of these planets in days. planet orbital period (days) a 600 b 80 c 1,000 d 500 e 100 move each planet to its orbit in the system.
Answers: 3

Chemistry, 23.06.2019 15:00
Food can lose electrons to air. this loss of electrons can create free radicals that destroy chemical bonds, hence spoiling the food. which term describes this process?
Answers: 1
You know the right answer?
The molar mass of cadmium chloride is 183 g. what mass of cdcl2 would be present in 10.0 ml of 9 m s...
Questions

Mathematics, 26.01.2021 06:00

History, 26.01.2021 06:00

Mathematics, 26.01.2021 06:00

English, 26.01.2021 06:00

Mathematics, 26.01.2021 06:00


Mathematics, 26.01.2021 06:00




Mathematics, 26.01.2021 06:00


Computers and Technology, 26.01.2021 06:00

Mathematics, 26.01.2021 06:00

Biology, 26.01.2021 06:00



Mathematics, 26.01.2021 06:00

French, 26.01.2021 06:00