
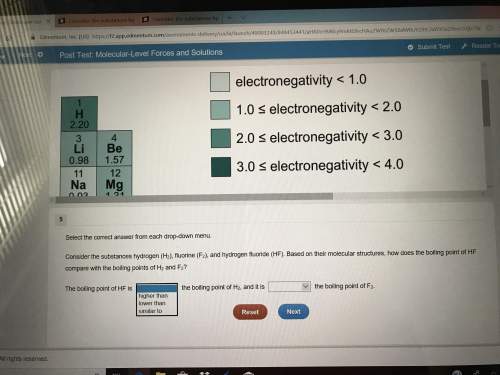
Plz don’t have much time left(picture included)select the correct answer from each drop-down menu. consider the substances hydrogen (h2), fluorine (f2), and hydrogen fluoride (hf). based on their molecular structures, how does the boiling point of hf compare with the boiling points of h2 and f2? the boiling point of hf is the boiling point of h2, and it is the boiling point of f2second set of opinions is the same

Answers: 3
Another question on Chemistry

Chemistry, 22.06.2019 09:20
Which of these statements explains the difference between nuclear binding energy and the strong nuclear force ?
Answers: 3

Chemistry, 22.06.2019 11:00
Which statement is true about hcl? (5 points) select one: a. it is a salt because it increases the concentration of metallic ions. b. it is a salt because it is formed by the reaction of an acid and a base. c. it is an acid because it increases the concentration of hydroxyl ions. d. it is an acid because it increases the concentration of hydronium ions.
Answers: 1

Chemistry, 22.06.2019 12:40
Quiz1. which physical state of nitrogen has the highest entropy? a solid© b gasoc liquid
Answers: 1

Chemistry, 22.06.2019 17:00
Complete each row of the table below by filling in the missing prefix or missing exponent.
Answers: 1
You know the right answer?
Plz don’t have much time left(picture included)select the correct answer from each drop-down menu....
Questions


World Languages, 26.02.2022 14:20


English, 26.02.2022 14:20


Business, 26.02.2022 14:20


Social Studies, 26.02.2022 14:20



Social Studies, 26.02.2022 14:20


Chemistry, 26.02.2022 14:20




English, 26.02.2022 14:20

Mathematics, 26.02.2022 14:20

Mathematics, 26.02.2022 14:20

Mathematics, 26.02.2022 14:30



