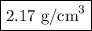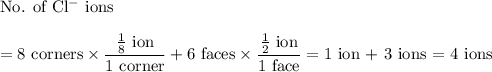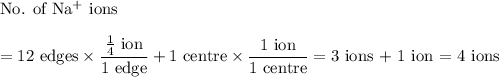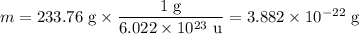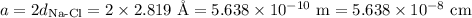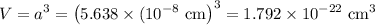Chemistry, 24.06.2019 18:10 makikorising1226
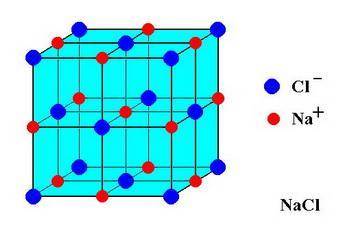
In sodium chloride, the distance between the center of the sodium ion and the centerof an adjacent chloride ion is 2.819 angstroms. calculate the density in g/cm3of an ideal nacl crystal from this information and what you learned from this lab. hints: to calculate mass, determine how many equivalent ions are in a unit cell. to determinevolume of the unit cell, start by determining the length of on side of the unit cell.

Answers: 2
Another question on Chemistry


Chemistry, 22.06.2019 09:50
What are four significant sources of ghgs that come from wostem washington?
Answers: 2

Chemistry, 22.06.2019 10:50
How many liters of oxygen gas, at standard temperature and pressure, will react with 35.8 grams of iron metal? 4 fe (s) + 3 o₂ (g) → 2 fe₂o₃ (s)
Answers: 2

Chemistry, 22.06.2019 11:00
Which statement correctly identifies the scientific question and describes why the question is scientific? question 1 refers to the supernatural.question 2 reflects a moral or social value.question 3 refers to something that can be measured.question 4 reflects a question that can’t be observed.
Answers: 1
You know the right answer?
In sodium chloride, the distance between the center of the sodium ion and the centerof an adjacent c...
Questions

History, 08.01.2021 18:30






Spanish, 08.01.2021 18:30

Mathematics, 08.01.2021 18:30


English, 08.01.2021 18:30


Mathematics, 08.01.2021 18:30



Mathematics, 08.01.2021 18:30




Mathematics, 08.01.2021 18:30