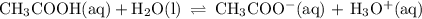Chemistry, 24.06.2019 18:10 terrieldixon
Consider a buffer solution containing ch3cooh and ch3coo-, with an equilibrium represented by: ch3cooh(aq) + h2o (l) ←→ h3o+ (aq) + ch3coo- (aq) describe what occurs if a strong acid such as hno3 is added to the system, including an explanation of the direction of the equilibrium shift. describe what occurs if a strong base such as koh is added, including an explanation of the direction of the equilibrium shift.

Answers: 3
Another question on Chemistry

Chemistry, 22.06.2019 00:00
Draw the skeletal structures of two different molecules that are each made of 5 carbon atoms and 12 hydrogen atoms.
Answers: 1


Chemistry, 22.06.2019 04:00
Gymnast always perform on padded mats. how does the mats protect the gymnast
Answers: 2

Chemistry, 22.06.2019 08:00
Define dew point. i am writing this part to be able to ask the question
Answers: 1
You know the right answer?
Consider a buffer solution containing ch3cooh and ch3coo-, with an equilibrium represented by: ch3c...
Questions

SAT, 15.01.2021 07:00


English, 15.01.2021 07:00

History, 15.01.2021 07:00



History, 15.01.2021 07:00

Mathematics, 15.01.2021 07:00

Mathematics, 15.01.2021 07:00

Mathematics, 15.01.2021 07:00


Mathematics, 15.01.2021 07:00

History, 15.01.2021 07:00

Geography, 15.01.2021 07:00



Mathematics, 15.01.2021 07:00

Mathematics, 15.01.2021 07:00

Mathematics, 15.01.2021 07:00

History, 15.01.2021 07:00