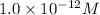Chemistry, 24.06.2019 15:40 dashawn3rd55
The ph of a solution is 2.0. which statement is correct? useful formulas include [h304)= 107, oh-1- 10+00h ph+poh = 14 and (h20-oh-] = 10-14 the poh of the solution is 12.0. the concentration of oh-ions is 1.0 x 10-2 m. the concentration of h3o+ ions is 100.0 m. the poh of the solution is 16.0.

Answers: 1
Another question on Chemistry

Chemistry, 21.06.2019 17:50
Which best describes why nh4+ can form an ionic bond with cl-?
Answers: 3

Chemistry, 22.06.2019 08:30
Which of the following would be an accurate picture of the earth during the summer time of the northern hemisphere?
Answers: 1


Chemistry, 22.06.2019 19:00
Which is the solubility product expression for caf2(s)?  [ca2+]/[f–]2  [ca2+][f2–]  [ca]+[f]2  [ca2+][f–]2
Answers: 3
You know the right answer?
The ph of a solution is 2.0. which statement is correct? useful formulas include [h304)= 107, oh-1-...
Questions

Mathematics, 08.06.2021 06:00

Arts, 08.06.2021 06:00

Mathematics, 08.06.2021 06:00

Social Studies, 08.06.2021 06:00



Mathematics, 08.06.2021 06:00

Mathematics, 08.06.2021 06:00

Social Studies, 08.06.2021 06:00

English, 08.06.2021 06:00

Social Studies, 08.06.2021 06:00


Mathematics, 08.06.2021 06:00

Mathematics, 08.06.2021 06:00


Computers and Technology, 08.06.2021 06:00


English, 08.06.2021 06:00


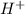 concentration.
concentration.
![pH=-\log [H^+]](/tpl/images/0012/3146/37e81.png)
![2=-\log [H^+]](/tpl/images/0012/3146/97dc6.png)
![[H^+]=0.01M](/tpl/images/0012/3146/8ae83.png)
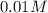
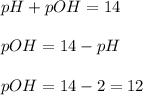
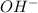 concentration.
concentration.
![pOH=-\log [OH^-]](/tpl/images/0012/3146/1fac1.png)
![12=-\log [OH^-]](/tpl/images/0012/3146/d45ca.png)
![[OH^-]=1.0\times 10^{-12}M](/tpl/images/0012/3146/83579.png)