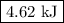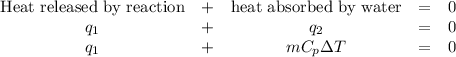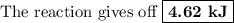When 40.0 ml of 1.00 m h2so4 is added to 80.0 ml of 1.00 m naoh at 20.00°c in a coffee cup calorimeter, the temperature of the aqueous solution increases to 29.20°c. if the mass of the solution is 120.0 g and the specific heat of the calorimeter and solution is 4.184 j/g • °c, how much heat is given off in the reaction? (ignore the mass of the calorimeter in the calculation.) use q=mcp(tiangle)t 4.62 kj 10.0 kj 14.7 kj 38.5 kj

Answers: 2
Another question on Chemistry

Chemistry, 20.06.2019 18:04
The volume of a sphere is given by v= (4/3) pi r cubed, where r is the radius. compute the volume of a sphere with a radius of 117pm. state your answer in units of cubed.
Answers: 1

Chemistry, 22.06.2019 01:00
Which part of a feedback mechanism is able to monitor the conditions outside of cells and usually uses nerve cells to relay this information to an intergrating center
Answers: 2

Chemistry, 22.06.2019 05:00
In 1901, thomas edison invented the nickel-iron battery. the following reaction takes place in the battery. fe(s) + 2 nio(oh)(s) + 2 h2o(l) fe(oh)2(s) + 2 ni(oh)2(aq) how many mole of fe(oh)2, is produced when 5.35 mol fe and 7.65 mol nio(oh) react?
Answers: 1

Chemistry, 22.06.2019 07:00
Indicate whether the specified alkyl halides will form primarily substitution products, only elimination products, both substitution and elimination products, or no products when they react with sodium methoxide. 1-bromobutane 1-bromo-2-methylpropane 2-bromobutane 2-bromo-2-methylpropane
Answers: 2
You know the right answer?
When 40.0 ml of 1.00 m h2so4 is added to 80.0 ml of 1.00 m naoh at 20.00°c in a coffee cup calorimet...
Questions

English, 02.04.2021 08:30


Mathematics, 02.04.2021 08:30

Mathematics, 02.04.2021 08:30



Biology, 02.04.2021 08:30


Mathematics, 02.04.2021 08:30

Mathematics, 02.04.2021 08:30

Mathematics, 02.04.2021 08:30


Mathematics, 02.04.2021 08:30


SAT, 02.04.2021 08:30



Biology, 02.04.2021 08:30