

Answers: 1
Another question on Chemistry

Chemistry, 22.06.2019 06:00
In 1901, thomas edison invented the nickel-iron battery. the following reaction takes place in the battery. fe(s) + 2 nio(oh)(s) + 2 h2o(l) fe(oh)2(s) + 2 ni(oh)2(aq) how many mole of fe(oh)2, is produced when 5.35 mol fe and 7.65 mol nio(oh) react?
Answers: 3

Chemistry, 22.06.2019 08:30
Identify one disadvantage to each of the following models of electron configuration: -dot structures -arrow and line diagrams -written electron configurations type in your answer below. (answer) -dot structures do not show the distribution of electrons in orbitals and take up a lot of space. -arrow and line diagrams take up a lot of space and make it difficult to count electrons. -written configurations make it easy to lose count of electrons and do not show the distribution of electrons in orbitals.
Answers: 3


Chemistry, 22.06.2019 21:00
Read "who built the pyramids? ”. leave this link open while you answer the questions throughout the assignment. give at least two reasons why some people claim the pyramids of giza were constructed by aliens.
Answers: 1
You know the right answer?
Calculate the energy in calories required to produce, from neutral he atoms,1 mole of he+ ions and h...
Questions

Mathematics, 28.10.2020 07:40

Social Studies, 28.10.2020 07:40



Mathematics, 28.10.2020 07:40



Mathematics, 28.10.2020 07:50

Mathematics, 28.10.2020 07:50

Mathematics, 28.10.2020 07:50

Mathematics, 28.10.2020 07:50



Mathematics, 28.10.2020 07:50


Mathematics, 28.10.2020 07:50

Mathematics, 28.10.2020 07:50

Social Studies, 28.10.2020 07:50


![\Delta E = R_{H}[\frac{1}{n^{2}_{i}} - \frac{1}{n^{2}_{f}}]](/tpl/images/0010/5843/a1c6b.png)
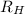 = Reydberg's constant =
= Reydberg's constant =  per meter
per meter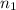 = 1 and
= 1 and 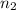 =
= 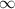
![1.09 \times 10^{7}[\frac{1}{(1)^{2}} - \frac{1}{\infty}}]](/tpl/images/0010/5843/d56c8.png) J
J ![1.09 \times 10^{7}[1 - 0}]](/tpl/images/0010/5843/bc35e.png) J
J ![1.09 \times 10^{7}]](/tpl/images/0010/5843/17892.png) J
J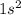 . Thus, energy required to produce
. Thus, energy required to produce 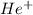 and
and  will be the same.
will be the same.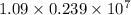 J
J cal
cal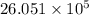 cal
cal

