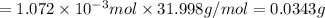Chemistry, 17.01.2020 02:31 tommyaberman
The henry's law constant (kh) for o2 in water at 20°c is 1.28 × 10−3 mol/(l·atm). (a) how many grams of o2 will dissolve in 4.00 l of h2o that is in contact with pure o2 at 1.00 atm? g o2 (b) how many grams of o2 will dissolve in 4.00 l of h2o that is in contact with air where the partial pressure of o2 is 0.209 atm?

Answers: 2
Another question on Chemistry

Chemistry, 21.06.2019 13:00
What is the ph of a solution with a 1.50 × 10−9 m hydroxide ion concentration?
Answers: 3

Chemistry, 23.06.2019 00:30
You are attempting to recrystallize a crude product mixture. you add the appropriate amount of hot solvent and are allowing the solution to slowly cool to room temperature. however, at room temperature no crystals have appeared, which of the following methods should be used to induce crystallization? choose all correct answers. a) place the flask in an ice bath. b) swirl the contents of the flask. c) add a small seed crystal of the desired product. d) scratch the inside of the glassware using a stir rod. it can be multiple choices
Answers: 3

Chemistry, 23.06.2019 07:00
How does science use models to gain a better understanding of concepts?
Answers: 1

Chemistry, 23.06.2019 15:30
Light travels through space at 186,282 miles per second and it takes about 1.3 seconds for light to travel from the moon to earth. which of the following is the correct method of finding the distance, in miles, between the moon and earth? add 186,282 and 1.3 divide 186,282 by 1.3 multiply 186,282 by 1.3 subtract 1.3 from 186,282
Answers: 1
You know the right answer?
The henry's law constant (kh) for o2 in water at 20°c is 1.28 × 10−3 mol/(l·atm). (a) how many grams...
Questions

Mathematics, 27.01.2021 22:40


Chemistry, 27.01.2021 22:40



Mathematics, 27.01.2021 22:40


Social Studies, 27.01.2021 22:40




Mathematics, 27.01.2021 22:40

Chemistry, 27.01.2021 22:40

Health, 27.01.2021 22:40




Mathematics, 27.01.2021 22:40

Mathematics, 27.01.2021 22:40

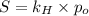
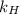 = Henry's law constant
= Henry's law constant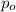 = Partial pressure of a gas
= Partial pressure of a gas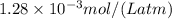
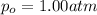
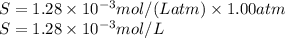
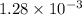 moles of oxygen gas in 1 liter of water
moles of oxygen gas in 1 liter of water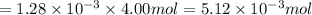
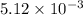 moles of oxygen gas:
moles of oxygen gas: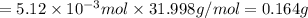
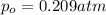
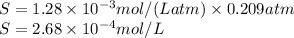
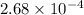 moles of oxygen gas in 1 liter of water
moles of oxygen gas in 1 liter of water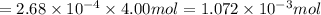
 moles of oxygen gas:
moles of oxygen gas: