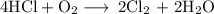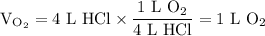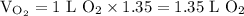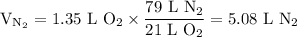)in the deacon process for the manufacture of chlorine, hcl and o2 react to form cl2 and h2o. sufficient air (21 mole% o2, 79% n2) is fed to provide 35% excess oxygen, and the fractional conversion of hcl is 85%. calculate the mole fractions of the product stream components.

Answers: 1
Another question on Chemistry

Chemistry, 22.06.2019 04:00
Electric charge is what ? a. kinetic energy b. radiation c. discovery d. electricity
Answers: 1

Chemistry, 22.06.2019 09:40
In the lab, ammonia was mixed with water to form ammonium hydroxide. what is/are the reactant(s)? o water and ammonia o ammonia o ammonium hydroxide need
Answers: 2


Chemistry, 22.06.2019 17:30
Upon decomposition, one sample of magnesium fluoride produced 1.65 kg of magnesium and 2.56 kg of fluorine. a second sample produced 1.32 kg of magnesium. part a how much fluorine (in grams) did the second sample produce?
Answers: 2
You know the right answer?
)in the deacon process for the manufacture of chlorine, hcl and o2 react to form cl2 and h2o. suffic...
Questions

Physics, 29.06.2020 18:01

Mathematics, 29.06.2020 18:01


Medicine, 29.06.2020 18:01

Mathematics, 29.06.2020 18:01

Mathematics, 29.06.2020 18:01

Mathematics, 29.06.2020 18:01

Medicine, 29.06.2020 18:01

Social Studies, 29.06.2020 18:01

Mathematics, 29.06.2020 18:01

Mathematics, 29.06.2020 18:01

Mathematics, 29.06.2020 18:01


Mathematics, 29.06.2020 18:01


Chemistry, 29.06.2020 18:01

Mathematics, 29.06.2020 18:01


Mathematics, 29.06.2020 18:01

English, 29.06.2020 18:01