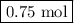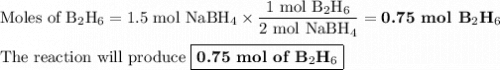Chemistry, 23.06.2019 07:20 msladycie8831
F1.5 mol of nabh4 react, how many moles of b2h6 are formed? 2 nabh4(aq) + h2so4(aq) → 2 h2(g) + na2so4(aq) + b2h6(g)

Answers: 1
Another question on Chemistry

Chemistry, 22.06.2019 02:30
If a 12-v battery is connected to a circuit that has a current of 3.0 a, what is the total resistance in the circuit? 36 ohms 4 ohms 0.25 ohms
Answers: 1

Chemistry, 22.06.2019 09:30
1. explain hydrogen peroxide, h 2 o 2 properties and decomposition reaction. 2. describe how each of the following natural cycles plays a part in earth’s climate system. (a) the water cycle (b) the carbon cycle
Answers: 1

Chemistry, 23.06.2019 16:30
Boron has an average atomic mass of 10.81. one isotope of boron has a mass of 10.012938 and a relative abundance of 19.80 percent . the other isotope has a relative abundance of 80.20 percent what is the mass of that isotope? report two decimal places
Answers: 1

You know the right answer?
F1.5 mol of nabh4 react, how many moles of b2h6 are formed? 2 nabh4(aq) + h2so4(aq) → 2 h2(g) + na2...
Questions

Law, 04.12.2020 01:00



Social Studies, 04.12.2020 01:00

English, 04.12.2020 01:00

History, 04.12.2020 01:00

English, 04.12.2020 01:00

Mathematics, 04.12.2020 01:00

Mathematics, 04.12.2020 01:00


Chemistry, 04.12.2020 01:00

Social Studies, 04.12.2020 01:00

Biology, 04.12.2020 01:00




Mathematics, 04.12.2020 01:00

Mathematics, 04.12.2020 01:00

Mathematics, 04.12.2020 01:00

Mathematics, 04.12.2020 01:00