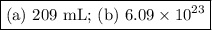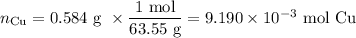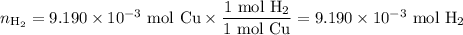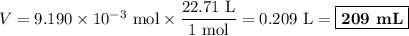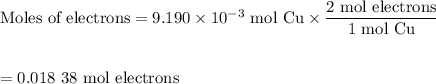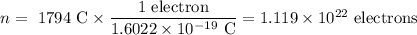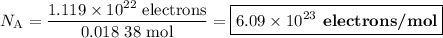An acidified solution was electrolyzed using copper electrodes. a constant current of 1.18 a caused the anode to lose 0.584 g after 1.52 ✕ 103 s. (a) what is the gas produced at the cathode and what is its volume at stp? name of gas volume of gas webassign will check your answer for the correct number of significant figures. l (b) given that the charge of an electron is 1.6022 ✕ 10−19 c, calculate avogadro's number. assume that copper is oxidized to cu2+ ions.

Answers: 3
Another question on Chemistry

Chemistry, 22.06.2019 00:00
Which type of bonding involves the complete transfer of a valence electron from a less electrogrative atom to a more electronegative one
Answers: 1

Chemistry, 22.06.2019 04:00
Seltzer water is created by placing water under pressure with carbon dioxide gas. which of the following statements best describe seltzer water: a. the solution will be slightly acidic b. the solution will be slightly basic. the solution will be strongly acidic. d. the solution will be strongly basic. e. the solution will be neutral
Answers: 3

Chemistry, 22.06.2019 16:50
Answer asap need by wednesday morning calculate the ph of 0.036m naoh best answer will be brainliest
Answers: 3

Chemistry, 23.06.2019 00:50
Which of the following warnings would an agricultural chemist tell a farmer who wants to recycle his or her own ammonia? recycling ammonia is a difficult process that sometimes takes weeks. recycling ammonia requires a degree in biochemistry or a related field. recycling ammonia can be harmful because it is highly flammable and toxic. recycling ammonia costs too much money considering the price of the necessary chemicals.
Answers: 1
You know the right answer?
An acidified solution was electrolyzed using copper electrodes. a constant current of 1.18 a caused...
Questions

Health, 06.05.2021 17:10


Mathematics, 06.05.2021 17:10



English, 06.05.2021 17:10

Mathematics, 06.05.2021 17:10

Mathematics, 06.05.2021 17:10


English, 06.05.2021 17:10

English, 06.05.2021 17:10




Mathematics, 06.05.2021 17:10

Chemistry, 06.05.2021 17:10

Mathematics, 06.05.2021 17:10

Physics, 06.05.2021 17:10