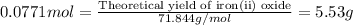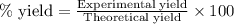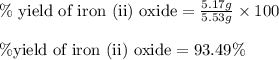Chemistry, 26.10.2019 11:43 Kathryn014
For the following reaction, 4.31 grams of iron are mixed with excess oxygen gas . the reaction yields 5.17 grams of iron(ii) oxide . iron ( s ) + oxygen ( g ) iron(ii) oxide ( s ) what is the theoretical yield of iron(ii) oxide ? 21.6 grams what is the percent yield for this reaction ? 85 %

Answers: 1
Another question on Chemistry

Chemistry, 22.06.2019 10:30
Astudent reacts 13 moles of iron with 21 moles of oxygen according to the following equation:
Answers: 2

Chemistry, 22.06.2019 12:00
Under normal conditions, describe how increasing the temperatures effects the solubility of a typical salt
Answers: 1

Chemistry, 22.06.2019 20:00
The volume of a single vanadium atom is 9.29×10-24 cm3. what is the volume of a vanadium atom in microliters?
Answers: 3

You know the right answer?
For the following reaction, 4.31 grams of iron are mixed with excess oxygen gas . the reaction yield...
Questions

Physics, 08.01.2021 03:10

English, 08.01.2021 03:10



Mathematics, 08.01.2021 03:10


Advanced Placement (AP), 08.01.2021 03:10



Mathematics, 08.01.2021 03:10

History, 08.01.2021 03:10


Mathematics, 08.01.2021 03:10

Mathematics, 08.01.2021 03:10

Biology, 08.01.2021 03:10



Mathematics, 08.01.2021 03:10


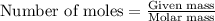 ....(1)
....(1)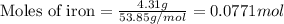
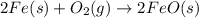
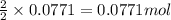 of iron (ii) oxide
of iron (ii) oxide