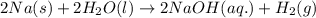Chemistry, 21.06.2019 15:50 algahimnada
In a popular classroom demonstration, solid sodium is added to liquid water and reacts to produce hydrogen gas and aqueous sodium hydroxide. part a write a balanced chemical equation for this reaction. express your answer as a chemical equation. identify all of the phases in your answer.

Answers: 3
Another question on Chemistry

Chemistry, 21.06.2019 23:30
Start an single atom tab. observe the decay of polonium-211. after each decay, press the reset nucleus button to watch the process again. write a description of alpha decay for po-211
Answers: 2

Chemistry, 22.06.2019 09:00
Particles vibrate in a rigid structure and do not move relative to their neighbors.
Answers: 1


Chemistry, 22.06.2019 12:40
Consider the directing effects of the substituents on salicylamide and predict the possible structures of the iodination products. which do you think will be the major product?
Answers: 1
You know the right answer?
In a popular classroom demonstration, solid sodium is added to liquid water and reacts to produce hy...
Questions


Biology, 12.10.2020 16:01


English, 12.10.2020 16:01

English, 12.10.2020 16:01

Mathematics, 12.10.2020 16:01

Social Studies, 12.10.2020 16:01

Mathematics, 12.10.2020 16:01

Mathematics, 12.10.2020 16:01





Mathematics, 12.10.2020 16:01



History, 12.10.2020 16:01

English, 12.10.2020 16:01