
Chemistry, 21.06.2019 14:10 yellowmiki6647
The rock in a lead ore deposit contains 89 % pbs by mass. how many kilograms of the rock must be processed to obtain 1.5 kg of pb?

Answers: 1
Another question on Chemistry

Chemistry, 21.06.2019 16:00
If the same amount of cacl2 is added to equal volumes of water and maple syrup, which will have the higher temperature?
Answers: 1

Chemistry, 22.06.2019 01:20
Match the acid base pairs by arranging the acid name with the conjugate base formula. hydrogen carbonate hydrogen phosphate carbonic acid read water sulfuric acid phosphoric acid a. co32- b. hso4- c. hco3- d. po43- e. h2po4- f. oh-
Answers: 1

Chemistry, 22.06.2019 09:30
In 2002, the rare earth elements mine in mountain pass, california was closed because
Answers: 1

Chemistry, 22.06.2019 17:50
Cryolite, na3alf6(s), an ore used in the production of aluminum, can be synthesized using aluminum oxide. start this question by first balance the chemical equation.1.) balance the equation: - alo3(s)+naoh(l)+hf(> na3alf6+h2o(g). 2.) if 17.5 kilograms of al2o3(s), 51.4 kilograms of naoh(l), and 51.4 kilograms of hf(g) react completely, how many kilograms of cryolite will be produced? 3.)which reactants will be in excess, (al2o3, naoh, or hf) 4.)what is the total mass of the excess reactants left over after the reaction is complete in kg?
Answers: 2
You know the right answer?
The rock in a lead ore deposit contains 89 % pbs by mass. how many kilograms of the rock must be pro...
Questions

Mathematics, 06.01.2022 04:40


History, 06.01.2022 04:40

SAT, 06.01.2022 04:40

Mathematics, 06.01.2022 04:40




Mathematics, 06.01.2022 04:40

Medicine, 06.01.2022 04:40


Mathematics, 06.01.2022 04:40

English, 06.01.2022 04:40

Mathematics, 06.01.2022 04:40


Mathematics, 06.01.2022 04:40

Mathematics, 06.01.2022 04:40


Geography, 06.01.2022 04:40

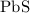 . There will be 890 grams of
. There will be 890 grams of 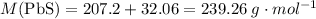 .
.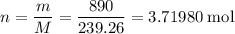 .
.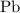 in each mole of
in each mole of 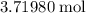 of
of 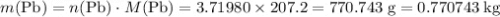 .
.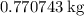 of lead
of lead 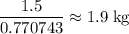 .
.

