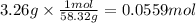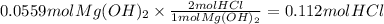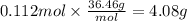Chemistry, 16.10.2019 23:30 wiljoystoltz253
Magnesium hydroxide, the active ingredient in milk of magnesia, neutralizes stomach acid, primarily hcl, according to the reaction mg(oh)2(aq)+2hcl(aq)→2h2o(l)+mgcl2( aq) what mass of hcl, in grams, is neutralized by a dose of milk of magnesia containing 3.26 g of mg(oh)2? express the mass in grams to three significant figures.

Answers: 1
Another question on Chemistry

Chemistry, 22.06.2019 05:40
Why did southern business leaders want to increase the number of slaves
Answers: 1

Chemistry, 22.06.2019 06:00
An atom of lithium (li) and an atom of chlorine (cl) engage in a chemical reaction. which correctly describes the structure of the resulting chemical compound? hint: consider the class of each element. the chemical compound will have a network structure. the chemical compound will have triple bonds. the chemical compound will have a ball-and-stick structure. the chemical compound will have double bonds.
Answers: 2

Chemistry, 22.06.2019 09:50
Although respiratory organs vary across different organisms, they all contain respiratory surfaces that have a large surface area and are extremely thin. explain why having an extremely thin respiratory surface with a large surface area is advantageous for the process of gas exchange
Answers: 1

Chemistry, 22.06.2019 14:00
How does the presence of oxygen affect the chemical pathways used to extract energy from glucose?
Answers: 3
You know the right answer?
Magnesium hydroxide, the active ingredient in milk of magnesia, neutralizes stomach acid, primarily...
Questions

Mathematics, 23.04.2020 23:04

SAT, 23.04.2020 23:04


Biology, 23.04.2020 23:04

Social Studies, 23.04.2020 23:04


Mathematics, 23.04.2020 23:04

Mathematics, 23.04.2020 23:04


Advanced Placement (AP), 23.04.2020 23:04

English, 23.04.2020 23:04

English, 23.04.2020 23:04


Mathematics, 23.04.2020 23:04


Biology, 23.04.2020 23:04


Mathematics, 23.04.2020 23:04

Mathematics, 23.04.2020 23:04

Biology, 23.04.2020 23:04