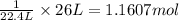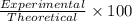Chemistry, 25.12.2019 11:31 jamarstand
Hydrogen gas (a potential future fuel) can be formed by the reaction of methane with water according to the following equation: ch4(g)+h2o(g)→co(g)+3h2(g) in a particular reaction, 26.0 l of methane gas (measured at a pressure of 734 torr and a temperature of 25 ∘c) is mixed with 23.0 l of water vapor (measured at a pressure of 700 torr and a temperature of 125 ∘c). the reaction produces 26.0 l of hydrogen gas measured at stp. part a what is the percent yield of the reaction? %

Answers: 2
Another question on Chemistry

Chemistry, 22.06.2019 00:00
The p sub shell can hold up to 8 electrons in an atom. true or false?
Answers: 1

Chemistry, 22.06.2019 14:30
Amixture that has two or more substances that are spread out evenly is called a. compound b. heterogeneous c. substance d. homogeneous
Answers: 1

Chemistry, 22.06.2019 20:10
Suppose you mix one mole of sulfuric acid (h2so4) with 1 mole of sodium hydroxide(naoh). why does the ph of the solution remain below 7? ( explain so i can get better understanding! )
Answers: 2

Chemistry, 22.06.2019 21:20
Phosgene (carbonyl chloride), cocl2, is an extremely toxic gas that is used in manufacturing certain dyes and plastics. phosgene can be produced by reacting carbon monoxide and chlorine gas at high temperatures: co(g) cl2(g)⇌cocl2(g) carbon monoxide and chlorine gas are allowed to react in a sealed vessel at 477 ∘c . at equilibrium, the concentrations were measured and the following results obtained: gas partial pressure (atm) co 0.830 cl2 1.30 cocl2 0.220 what is the equilibrium constant, kp, of this reaction
Answers: 2
You know the right answer?
Hydrogen gas (a potential future fuel) can be formed by the reaction of methane with water according...
Questions




Mathematics, 23.06.2019 06:00






Biology, 23.06.2019 06:00


Biology, 23.06.2019 06:00


Chemistry, 23.06.2019 06:00



Mathematics, 23.06.2019 06:00



Mathematics, 23.06.2019 06:00

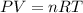
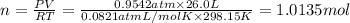
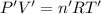
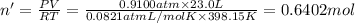
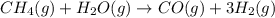
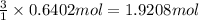 of hydrogen gas
of hydrogen gas