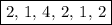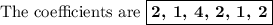In acidic solution, the nitrate ion can be used to react with a number of metal ions. one such reaction is no3−(aq)+sn2+(aq)→no2(aq)+sn4+(aq) since this reaction takes place in acidic solution, h2o(l) and h+(aq) will be involved in the reaction. places for these species are indicated by the blanks in the following restatement of the equation: no3−(aq)+sn2+(aq)+ −−−→no2(aq)+sn4+(aq)+ −−− part a what are the coefficients of the reactants and products in the balanced equation above? remember to include h2o(l) and h+(aq) in the appropriate blanks. your answer should have six terms. enter the equation coefficients in order separated by commas (e. g., 2,2,1,4,4,3). include coefficients of 1, as required, for grading purposes.

Answers: 2
Another question on Chemistry

Chemistry, 21.06.2019 22:30
Omg imgonnafailnfiedkla use complete sentences to explain how the mass of hydrogen is conserved during cellular respiration.
Answers: 1


Chemistry, 23.06.2019 18:30
Molecules of sugar are attracted to each other and form crystals. if you place sugar in water, the crystals break apart, but the molecules remain the same kind of sugar. what can you infer about the bonds between atoms in a sugar molecule compared with the forces that attract sugar molecules to each other?
Answers: 1

Chemistry, 23.06.2019 19:30
How has the scientific model of the atom changed over the centuries, and what new evidence led to the various changes in the model?
Answers: 1
You know the right answer?
In acidic solution, the nitrate ion can be used to react with a number of metal ions. one such react...
Questions

Mathematics, 28.11.2019 20:31

Mathematics, 28.11.2019 20:31


Spanish, 28.11.2019 20:31




Biology, 28.11.2019 20:31


Mathematics, 28.11.2019 20:31

Mathematics, 28.11.2019 20:31




Mathematics, 28.11.2019 20:31



English, 28.11.2019 20:31

Mathematics, 28.11.2019 20:31

History, 28.11.2019 20:31