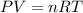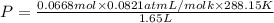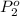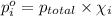Chemistry, 13.10.2019 01:10 sabrinamarie391
Agas mixture contains 1.20 g n2 and 0.77 g o2 in a 1.65-l container at 15 ∘c. part a calculate the mole fraction of n2. express your answer using two significant figures. x1 x 1 = nothing request answer part b calculate the mole fraction of o2. express your answer using two significant figures. x2 x 2 = nothing request answer part c calculate the partial pressure of n2. express your answer using two significant figures. p1 p 1 = nothing atm request answer part d calculate the partial pressure of o2. express your answer using two significant figures. p2 p 2 = nothing atm request answer provide feedback

Answers: 2
Another question on Chemistry


Chemistry, 22.06.2019 14:30
In water, a strong acid will break down into its component parts. a. completely b. partly c. never in water, a weak base will break down into its component parts. a. completely b. partly c. never
Answers: 2

Chemistry, 22.06.2019 17:40
Which statement about hf is true? it is zero for any compound in its standard state. it is positive when the bonds of the product store more energy than those of the reactants. it is negative when a compound forms from elements in their standard states. it is zero for any element that is in the liquid state.
Answers: 1

Chemistry, 22.06.2019 18:00
How is energy related to the change of state represented by the model? atoms gain energy as a solid changes to a liquid. atoms gain energy as a solid changes to a gas. atoms lose energy as a solid changes to a liquid. atoms lose energy as a solid changes to a gas.
Answers: 3
You know the right answer?
Agas mixture contains 1.20 g n2 and 0.77 g o2 in a 1.65-l container at 15 ∘c. part a calculate the m...
Questions



Physics, 11.02.2021 03:40



Mathematics, 11.02.2021 03:40

English, 11.02.2021 03:40

Mathematics, 11.02.2021 03:40


English, 11.02.2021 03:40

Health, 11.02.2021 03:40





Biology, 11.02.2021 03:40





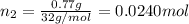

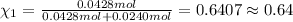

 = 0.0428 mol + 0.0240 mol = 0.0668 mol
= 0.0428 mol + 0.0240 mol = 0.0668 mol