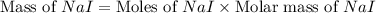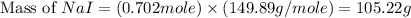Chemistry, 15.11.2019 14:31 ejfleck3655
Iodine is prepared both in the laboratory and commercially by adding cl2(g)cl2(g) to an aqueous solution containing sodium iodide. 2nai(aq)+cl2(g)⟶i2(s)+2nacl(aq) 2nai(aq)+cl2(g)⟶i2(s)+2nacl(aq) how many grams of sodium iodide, nai, nai, must be used to produce 89.1 g89.1 g of iodine, i2? i2? mass: g nai

Answers: 2
Another question on Chemistry

Chemistry, 22.06.2019 09:40
Apiece of copper has a temperature of 75.6 0c. when the metal is placed in 100.0 grams of water at 19.1 0c, the temperature rises by 5.5 0c. what is the mass of the metal?
Answers: 1

Chemistry, 22.06.2019 19:10
How does the atmosphere to make earth livable? check all that apply. causes the seasons contains oxygen provides warmth creates important nutrients blocks harmful energy from the sun plz like !
Answers: 2


Chemistry, 22.06.2019 19:30
To calculate percent by mass, use the equation below: calculate the percent by mass of each element. %n = % %h = % %o = %
Answers: 3
You know the right answer?
Iodine is prepared both in the laboratory and commercially by adding cl2(g)cl2(g) to an aqueous solu...
Questions

Chemistry, 05.02.2022 04:40


Mathematics, 05.02.2022 04:40

Mathematics, 05.02.2022 04:40



Mathematics, 05.02.2022 04:40

Physics, 05.02.2022 04:40


English, 05.02.2022 04:40

Mathematics, 05.02.2022 04:40


Biology, 05.02.2022 04:40







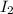 = 89.1 g
= 89.1 g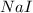 = 149.89 g/mole
= 149.89 g/mole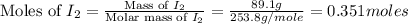
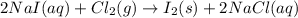
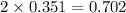 moles of
moles of