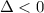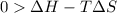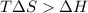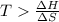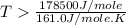For the decomposition of calcium carbonate, consider the following thermodynamic data (due to variations in thermodynamic values for different sources, be sure to use the given values in calculating your answer.): δh∘rxn 178.5kj/mol δs∘rxn 161.0j/(mol⋅k) calculate the temperature in kelvins above which this reaction is spontaneous.

Answers: 2
Another question on Chemistry

Chemistry, 21.06.2019 17:10
There are 6.022 x 10^23 atoms of hg in 1 mole of hg. the number of atoms in 4.5 moles of hg can be found by multiplying 4.5 by 6.022 x 10^23 a. 2.7 x 10^24 b. 27 x 10^23 c. 2.71 x10^24 d. 27.099 x 10^23
Answers: 3

Chemistry, 22.06.2019 00:00
Explain which group an element with the electron configuration 1s2 2s2 2p6 3s2 3p6 3d1 4s2 belongs to.
Answers: 3


Chemistry, 22.06.2019 22:30
Which is a characteristic of the electron sea model for metallic bonding? molecular orbitals overlap to produce bands. electrons flow easily between metal nuclei. electrons are in fixed positions in the orbitals. atomic nuclei are arranged in an irregular pattern.
Answers: 3
You know the right answer?
For the decomposition of calcium carbonate, consider the following thermodynamic data (due to variat...
Questions







Mathematics, 30.07.2020 20:01

Mathematics, 30.07.2020 20:01






Mathematics, 30.07.2020 20:01

History, 30.07.2020 20:01

Mathematics, 30.07.2020 20:01

Mathematics, 30.07.2020 20:01

Mathematics, 30.07.2020 20:01


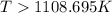
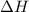 = 178.5 KJ/mole = 178500 J/mole
= 178.5 KJ/mole = 178500 J/mole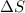 = 161.0 J/mole.K
= 161.0 J/mole.K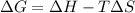
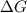 is negative or we can say that the value of
is negative or we can say that the value of