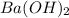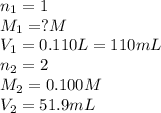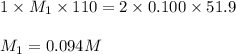Chemistry, 10.01.2020 11:31 mpzpowell7506
Consider the neutralization reaction 2hno3(aq)+ba(oh)2(aq)⟶2h2o(l)+ba(no 3)2(aq) 2hno3(aq)+ba(oh)2(aq)⟶2h2o(l)+ba(no 3)2(aq) a 0.110 l0.110 l sample of an unknown hno3hno3 solution required 51.9 ml51.9 ml of 0.100 m ba(oh)20.100 m ba(oh)2 for complete neutralization. what is the concentration of the hno3hno3 solution? concentration:

Answers: 1
Another question on Chemistry

Chemistry, 21.06.2019 15:50
Nitrogen dioxide reacts with water to form nitric acid and nitrogen monoxide according to the equation: 3no2(g)+h2o(l)→2hno3(l)+no(g) part a suppose that 4.2 mol no2 and 0.50 mol h2o combine and react completely. which reactant is in excess? express your answer as a chemical formula. nothing
Answers: 1

Chemistry, 22.06.2019 19:00
Sum of brother and sisters age is 26. four times the brothers age is subtracted from three times the sisters age, the difference is 8. what are the ages of the brother and sister?
Answers: 1

Chemistry, 22.06.2019 19:50
When the mercury level in a barometer decreases that atmospheric pressure has
Answers: 3

You know the right answer?
Consider the neutralization reaction 2hno3(aq)+ba(oh)2(aq)⟶2h2o(l)+ba(no 3)2(aq) 2hno3(aq)+ba(oh)2(a...
Questions



Physics, 21.11.2019 06:31

Spanish, 21.11.2019 06:31


Mathematics, 21.11.2019 06:31


English, 21.11.2019 06:31


English, 21.11.2019 06:31

Physics, 21.11.2019 06:31



English, 21.11.2019 06:31

English, 21.11.2019 06:31




Mathematics, 21.11.2019 06:31

 solution will be 0.094 M.
solution will be 0.094 M.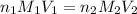
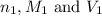 are the n-factor, molarity and volume of acid which is
are the n-factor, molarity and volume of acid which is 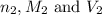 are the n-factor, molarity and volume of base which is
are the n-factor, molarity and volume of base which is