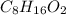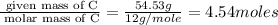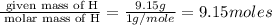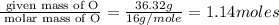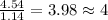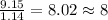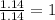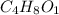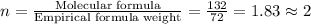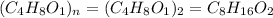Chemistry, 21.10.2019 16:50 arnold2619
Acompound is 54.53% c,54.53% c, 9.15% h,9.15% h, and 36.32% o36.32% o by mass. what is its empirical formula? insert subscripts as needed. empirical formula: chocho the molecular mass of the compound is 132 amu.132 amu. what is its molecular formula? insert subscripts as needed. molecular formula: cho

Answers: 3
Another question on Chemistry

Chemistry, 22.06.2019 03:30
Select the correct answer. when carbon dioxide dissolves in water, it sometimes reacts with water to form carbonic acid as in this balanced equation: co2 + h2o → h2co3. if 495 milliliters of carbon dioxide at 25°c and 101.3 kilopascals reacts with excess water, what is the theoretical yield of carbonic acid? use the periodic table and the ideal gas resource a. 0.889 g b. 1.10g c. 1.27g d. 2.02g what's the answer! quick!
Answers: 1

Chemistry, 22.06.2019 16:00
How does blood clotting prevent the entry of pathogens through cuts and wounds? answer asap,, this is due tomorrow. will mark as brainliest or whatever you call it : )
Answers: 2

Chemistry, 22.06.2019 20:10
What would happen to a volleyball left outside in the winter? o o o o a. it would expand. b. it would lose air. c. it would shrink. d. it would explode.
Answers: 2

Chemistry, 22.06.2019 21:20
Phosgene (carbonyl chloride), cocl2, is an extremely toxic gas that is used in manufacturing certain dyes and plastics. phosgene can be produced by reacting carbon monoxide and chlorine gas at high temperatures: co(g) cl2(g)⇌cocl2(g) carbon monoxide and chlorine gas are allowed to react in a sealed vessel at 477 ∘c . at equilibrium, the concentrations were measured and the following results obtained: gas partial pressure (atm) co 0.830 cl2 1.30 cocl2 0.220 what is the equilibrium constant, kp, of this reaction
Answers: 2
You know the right answer?
Acompound is 54.53% c,54.53% c, 9.15% h,9.15% h, and 36.32% o36.32% o by mass. what is its empirical...
Questions


History, 21.07.2019 12:30


Mathematics, 21.07.2019 12:30







Mathematics, 21.07.2019 12:30

History, 21.07.2019 12:30





Social Studies, 21.07.2019 12:30

English, 21.07.2019 12:30