Chemistry, 20.01.2020 18:31 DeonDub3106
Titration of a 20.0-ml sample of acid rain required 1.7 ml of 0.0811 m naoh to reach the end point. if we assume that the acidity of the rain is due to the presence of sulfuric acid, what was the concentration of sulfuric acid in this sample of rain?

Answers: 1
Another question on Chemistry

Chemistry, 22.06.2019 20:00
Suppose that some of the compound spilled out of the crucible after it was heated. would that cause the percent by mass of water in the compound determined by the experiment to be too low, too high, or unchanged? briefly explain your answer.
Answers: 1

Chemistry, 22.06.2019 23:30
Aweight lifter raises a 1600 n barbell to a height of 2.0 meters. how much work was done? w = fd a) 30 joules b) 3000 joules c) 320 joules d) 3200 joules
Answers: 2

Chemistry, 23.06.2019 15:50
Astable atom that has a large nucleus most likely contains 1. more neutrons than protons. 2.more protons than neutrons. 3.equal numbers of protons and neutrons. 4.changing numbers of protons and neutrons.
Answers: 1

Chemistry, 23.06.2019 16:30
Asample of calcium chloride (cacla) contains 100.2 grams of calcium (ca) and 177.3 grams of chlorine (co. what is the percent composition of chlorine 2.510% 36.10% 56.50% 63.89%
Answers: 2
You know the right answer?
Titration of a 20.0-ml sample of acid rain required 1.7 ml of 0.0811 m naoh to reach the end point....
Questions

History, 18.06.2020 06:57

Health, 18.06.2020 06:57




Mathematics, 18.06.2020 06:57

Mathematics, 18.06.2020 06:57

English, 18.06.2020 06:57

Mathematics, 18.06.2020 06:57







English, 18.06.2020 06:57




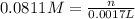
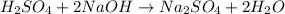
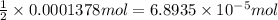 of sulfuric acid.
of sulfuric acid.