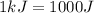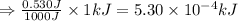Chemistry, 31.01.2020 22:02 ComicSans01
Suppose you are investigating the reaction: m(s) + 2 hcl(aq) → mcl2(aq) + h2(g). you weigh out a 0.295 gram piece of metal and combine it with 65 ml of 1.00 m hcl in a coffee-cup calorimeter. if the molar mass of the metal is 57.78 g/mol, and you measure that the reaction absorbed 104 j of heat, what is the enthalpy of this reaction in kj per mole of limiting reactant? enter your answer numerically to three significant figures in units of kj/mol.

Answers: 2
Another question on Chemistry

Chemistry, 21.06.2019 23:10
Which statement describes both homogeneous mixtures and heterogeneous mixtures?
Answers: 1


Chemistry, 22.06.2019 07:00
How heavy is thanos? a) 3000 lbs b) all of it c) the price of tea in china d) heavy enough
Answers: 2

Chemistry, 22.06.2019 10:40
If an area has high air pressure and low humidity, what type of weather will it most likely have? plz !
Answers: 1
You know the right answer?
Suppose you are investigating the reaction: m(s) + 2 hcl(aq) → mcl2(aq) + h2(g). you weigh out a 0....
Questions














Mathematics, 12.08.2019 20:30

Mathematics, 12.08.2019 20:30


Mathematics, 12.08.2019 20:30



Mathematics, 12.08.2019 20:30

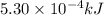
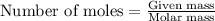 ....(1)
....(1)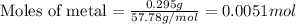
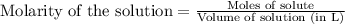
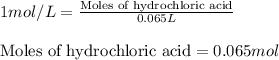
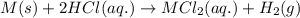
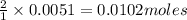 of hydrochloric acid.
of hydrochloric acid.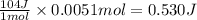 of heat.
of heat.