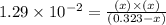Answers: 3
Another question on Chemistry

Chemistry, 21.06.2019 18:30
150.0 grams of asf3 were reacted with 180.0 g of ccl4 to produce ascl3 and ccl2f2. if the actual yield of ccl2f2 was 127 g, what is the percent yield?
Answers: 2

Chemistry, 23.06.2019 02:00
An alpha particle is: a hydrogen atom a nucleus of helium two neutrons an electron
Answers: 1

Chemistry, 23.06.2019 04:00
What are the names of these two interactions with cattle and how do they differ from each other
Answers: 3

Chemistry, 23.06.2019 05:00
He nucleus contains the cells genetic material in the form of dna. dna is organized into our chromosomes, which are made up of thousands of that determine our traits.
Answers: 1
You know the right answer?
The equilibrium constant, kc, for the following reaction is 1.29×10-2 at 600 k. cocl2(g) co(g) + cl2...
Questions

English, 25.09.2021 14:00

Physics, 25.09.2021 14:00


Mathematics, 25.09.2021 14:00

English, 25.09.2021 14:00

Advanced Placement (AP), 25.09.2021 14:00


Engineering, 25.09.2021 14:00

Mathematics, 25.09.2021 14:00

Spanish, 25.09.2021 14:00

Physics, 25.09.2021 14:00

Mathematics, 25.09.2021 14:00

Geography, 25.09.2021 14:00

History, 25.09.2021 14:00

Mathematics, 25.09.2021 14:00

Mathematics, 25.09.2021 14:00



Mathematics, 25.09.2021 14:00

History, 25.09.2021 14:00

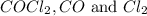 are, 0.2646, 0.0584 and 0.0584.
are, 0.2646, 0.0584 and 0.0584.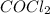 = 0.323 mole
= 0.323 mole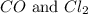 be, 'x'. So,
be, 'x'. So,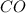 = x M
= x M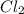 = x M
= x M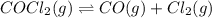
![K_c=\frac{[CO][Cl_2]}{[COCl_2]}](/tpl/images/0436/7314/59c52.png)