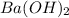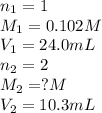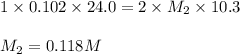Answers: 1
Another question on Chemistry


Chemistry, 22.06.2019 22:30
Essay-alternative energy sources research sources of energy that are being developed. write a report of 350-400 words discussing the information you learned concerning the development of various energy sources and the impact that you think they will have on your life. include sources cited at the end of your report using the mla format. follow the rubric guidelines. note that wikipedia is not an appropriate resource for a research paper. worth 99
Answers: 3

Chemistry, 23.06.2019 00:00
How many peaks will be present in a mass spectrum for brcl?
Answers: 1

Chemistry, 23.06.2019 03:00
In november 1987, a massive iceberg broke loose from the antartic ice mass and floated free in the ocean. the chunk of ice was estimated to be 98 mi long, 25 mi wide, and 750 ft thick. a typical backyard swimming pool contains about 24,000 gallons of water. how many of these pools could you fill from the water in this iceberg? (assume the iceberg is a rectangular solid of the above dimensions and consists of water only). express answer in scientific notation.
Answers: 3
You know the right answer?
An aqueous solution of barium hydroxide is standardized by titration with a 0.102 m solution of perc...
Questions

Chemistry, 15.03.2020 15:31

History, 15.03.2020 15:31

Mathematics, 15.03.2020 15:31



Chemistry, 15.03.2020 15:32

Mathematics, 15.03.2020 15:33

Mathematics, 15.03.2020 15:33

Biology, 15.03.2020 15:35





Mathematics, 15.03.2020 15:38

Geography, 15.03.2020 15:39


World Languages, 15.03.2020 15:41


Biology, 15.03.2020 15:43

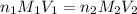
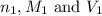 are the n-factor, molarity and volume of acid which is
are the n-factor, molarity and volume of acid which is 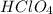
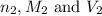 are the n-factor, molarity and volume of base which is
are the n-factor, molarity and volume of base which is