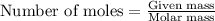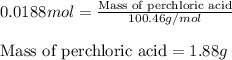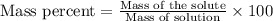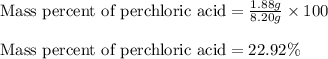Answers: 2
Another question on Chemistry

Chemistry, 22.06.2019 03:20
What is the ima of the 1 st class lever in the graphic given? 2 3 0.5
Answers: 1

Chemistry, 22.06.2019 09:00
This chart lists four kinds of polymers and their sources. what can be known about all four polymers, despite their differences? they come from living things. they share ionic carbon bonds. they are at least 100 monomers long. they are made of repeating subunits.
Answers: 3

Chemistry, 22.06.2019 15:20
Water is initially present in a state where its molecules are far apart. during a change of state, its molecules slow down. which change of state has most likely taken place? from a gas to a liquid from a liquid to a gas from a solid to a liquid from a gas to a plasma
Answers: 1

Chemistry, 23.06.2019 00:00
The empirical formula of a compound is ch2o and its mass is 120 amu/molecule, what is its formula?
Answers: 2
You know the right answer?
A8.20 g sample of an aqueous solution of perchloric acid contains an unknown amount of the acid. if...
Questions


Mathematics, 29.01.2021 16:40




Biology, 29.01.2021 16:40

English, 29.01.2021 16:40


Mathematics, 29.01.2021 16:40

Mathematics, 29.01.2021 16:40




Chemistry, 29.01.2021 16:40

Mathematics, 29.01.2021 16:40





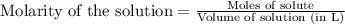
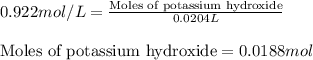
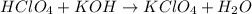
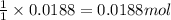 of perchloric acid.
of perchloric acid.