
Chemistry, 23.01.2020 04:31 caitlynnstokes
In an electroplating process, copper (ionic charge +2e, atomic weight 63.6 g/mol) is deposited using a current of 10.0 a. what mass of copper is deposited in 10.0 minutes? avogadro's number is 6.022 × 1023 molecules/mol and e = 1.60 × 10-19 c.

Answers: 3
Another question on Chemistry

Chemistry, 22.06.2019 00:30
What must happen before a body cell can begin mitotic cell division
Answers: 2

Chemistry, 22.06.2019 06:30
Type the correct answer in the box. spell all words correctly.what is the correct term for living the most sustainable life you can within your current circumstances? when your are being as sustainable as you can within your current lifestyle, you are said to be sustainability.
Answers: 3


Chemistry, 22.06.2019 20:00
Listenbase your answer to the question on the information below.nuclear radiation is harmful to living cells, particularly to fast-growing cells, such as cancer cells and blood cells. an external beam of the radiation emitted from a radioisotope can be directed on a small area of a person to destroy cancer cells within the body.cobalt-60 is an artificially produced radioisotope that emits gamma rays and beta particles. one hospital keeps a 100.0-gram sample of cobalt-60 in an appropriate, secure storage container for future cancer treatment.which choice represents the correct product for the beta decay of the co-60? fe-60ni-60fe-61ni-61
Answers: 2
You know the right answer?
In an electroplating process, copper (ionic charge +2e, atomic weight 63.6 g/mol) is deposited using...
Questions

Spanish, 01.10.2019 19:00


Health, 01.10.2019 19:00

Mathematics, 01.10.2019 19:00

Social Studies, 01.10.2019 19:00

English, 01.10.2019 19:00

Mathematics, 01.10.2019 19:00

Social Studies, 01.10.2019 19:00

Biology, 01.10.2019 19:00

Biology, 01.10.2019 19:00


English, 01.10.2019 19:00

Mathematics, 01.10.2019 19:00





History, 01.10.2019 19:00

Biology, 01.10.2019 19:00

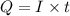

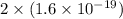
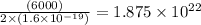 atoms
atoms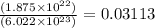 moles
moles



