
Chemistry, 28.01.2020 01:31 JessTaylr04
Aself-contained underwater breathing apparatus (scuba) uses canisters containing potassium superoxide. the superoxide consumes the co2 exhaled by a person and replaces it with oxygen. 4 ko2(s) + 2 co2(g) n 2 k2co3(s) + 3 o2(g) what mass of ko2, in grams, is required to react with 8.90 l of co2 at 22.0 °c and 767 mm hg

Answers: 3
Another question on Chemistry

Chemistry, 21.06.2019 22:30
200. ml of 3.00 m nacl solution is diluted to a final volume of 500. ml. what is the molarity of the final solution?
Answers: 2

Chemistry, 22.06.2019 07:30
All cells are made of four types of acids, lipids, proteins, and carbohydrates.
Answers: 1

Chemistry, 22.06.2019 10:00
Suppose the universe were completely empty except for one object-a solid sphere moving through space of 100 km/s. what sort of path would the object be moving in? explain your answer
Answers: 1

Chemistry, 22.06.2019 18:30
Which of the following nuclei would be the least stable a 2 protons, 2 neutrons b 1 proton 1 neutron c 1 proton 3 neutrons d 1 proton 2 neutrons
Answers: 3
You know the right answer?
Aself-contained underwater breathing apparatus (scuba) uses canisters containing potassium superoxid...
Questions


Mathematics, 24.11.2019 15:31

Physics, 24.11.2019 15:31


Physics, 24.11.2019 15:31

Mathematics, 24.11.2019 15:31







History, 24.11.2019 15:31


Mathematics, 24.11.2019 15:31

English, 24.11.2019 15:31

Mathematics, 24.11.2019 15:31

History, 24.11.2019 15:31

Biology, 24.11.2019 15:31

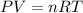
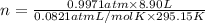
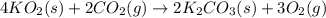
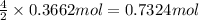 of potassium superoxide.
of potassium superoxide.


