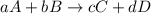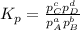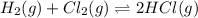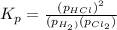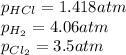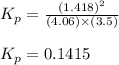Chemistry, 26.06.2019 03:20 elopezhilario6339
At a given temperature, 4.06 atm of h2 and 3.5 atm of cl2 are mixed and allowed to come to equilibrium. the equilibrium pressure of hcl is found to be 1.418 atm. calculate kp for the reaction at this temperature. h2(g) + cl2(g) < => 2 hcl(g)

Answers: 1
Another question on Chemistry

Chemistry, 21.06.2019 21:30
Put these processes of the water cycle in the correct order, starting at the point where the water is in the lake: 1. water evaporates into the atmosphere 2. rain, snow, or other precipitation falls 3. water collects into larger bodies of water 4. water vapor condenses into liquid water
Answers: 1



Chemistry, 23.06.2019 01:50
Ablock of aluminum is dropped into a graduated cylinder with an initial volume of water at 75ml and the volumes rises to 90ml. if the block has a mass of 40.5 g what is its density ?
Answers: 1
You know the right answer?
At a given temperature, 4.06 atm of h2 and 3.5 atm of cl2 are mixed and allowed to come to equilibri...
Questions


Mathematics, 12.05.2020 09:57


Mathematics, 12.05.2020 09:57

Mathematics, 12.05.2020 09:57


Business, 12.05.2020 09:57




English, 12.05.2020 09:57


History, 12.05.2020 09:57



Mathematics, 12.05.2020 09:57


English, 12.05.2020 09:57


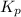 for the given chemical reaction is 0.1415
for the given chemical reaction is 0.1415