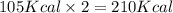Chemistry, 26.06.2019 03:40 ayoismeisjjjjuan
When nh3(g) reacts with n2o(g) to form n2(g) and h2o(g), 105 kcal of energy are evolved for each mole of nh3(g) that reacts. write a balanced equation for the reaction with an energy term in kcal as part of the equation.

Answers: 3
Another question on Chemistry


Chemistry, 22.06.2019 03:30
Adrop of acetone (nail polish remover) has a mass of 35 mg and a density of 0.788 g/cm3. what is its volume in cubic centimeters?
Answers: 3

Chemistry, 22.06.2019 10:30
Which describes fat? a: a carbohydrate that produces energy b: a nucleic acid that directs cell function c: a lipid that stores energy d: a protein that speeds up a chemical reaction
Answers: 1

Chemistry, 23.06.2019 00:10
In as 1°, 2°, 3°, or 4°. be to . : °b: °c: °d: ° : °b: °c: °d: ° : °b: °c: °d: °e: °f: °g: °h: ° : °b: °c: °d: °e: °f: °g: °h: °i: °
Answers: 3
You know the right answer?
When nh3(g) reacts with n2o(g) to form n2(g) and h2o(g), 105 kcal of energy are evolved for each mol...
Questions

Biology, 31.03.2021 16:10


Mathematics, 31.03.2021 16:10

Business, 31.03.2021 16:10


Mathematics, 31.03.2021 16:10

Mathematics, 31.03.2021 16:10




Mathematics, 31.03.2021 16:10

Mathematics, 31.03.2021 16:10


English, 31.03.2021 16:10




Mathematics, 31.03.2021 16:10

Mathematics, 31.03.2021 16:10

Mathematics, 31.03.2021 16:10

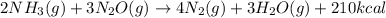
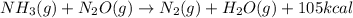
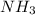 , the coefficient 3 is put before the
, the coefficient 3 is put before the 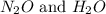 and the coefficient 4 is put before the
and the coefficient 4 is put before the 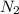 .
.