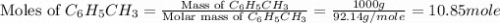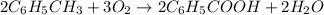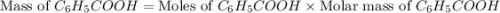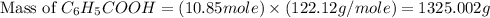Chemistry, 26.06.2019 04:10 samsavage4073
Toluene, c6h5ch3, is oxidized by air under carefully controlled conditions to benzoic acid, c6h5co2h, which is used to prepare the food preservative sodium benzoate, c6h5co2na. what is the percent yield of a reaction that converts 1.000 kg of toluene to 1.21 kg of benzoic acid?

Answers: 2
Another question on Chemistry

Chemistry, 21.06.2019 22:30
1. combine iron and copper (ii) sulfate solution. (hint: iron will form the iron (iii) ion) fe + cuso4 → 2. combine lead (ii) nitrate and potassium iodide solutions. pb(no3)2+ kl → 3. combine magnesium metal and hydrochloric acid solution. mg + hcl → 4. electrolysis (splitting) of water. h2o → 5. burning magnesium. mg + o2 →
Answers: 3

Chemistry, 22.06.2019 08:30
Which of the following would be an accurate picture of the earth during the summer time of the northern hemisphere?
Answers: 1

Chemistry, 22.06.2019 17:30
Which scenario is most similar to the type of collision that gas particles have according to kinetic molecular theory
Answers: 1

Chemistry, 22.06.2019 23:00
Which of your 24 wells had indications that a chemical reaction occurred? how were you able to tell that a chemical reaction occurred? which of your 24 wells had indications that a physical reaction occurred? how were you able to tell that a physical reaction occurred? report on both mixing and evaporation. make a general statement about whether your hypotheses were validated or rejected. must your hypotheses be correct for this to be a successful laboratory?
Answers: 3
You know the right answer?
Toluene, c6h5ch3, is oxidized by air under carefully controlled conditions to benzoic acid, c6h5co2h...
Questions


Biology, 09.02.2021 19:40

Mathematics, 09.02.2021 19:40


Mathematics, 09.02.2021 19:40



Mathematics, 09.02.2021 19:40

Social Studies, 09.02.2021 19:40

Mathematics, 09.02.2021 19:40

Mathematics, 09.02.2021 19:40



Spanish, 09.02.2021 19:40

Mathematics, 09.02.2021 19:40

Biology, 09.02.2021 19:40




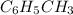 = 1 Kg = 1000 g
= 1 Kg = 1000 g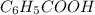 = 122.12 g/mole
= 122.12 g/mole