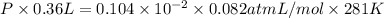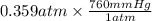Chemistry, 26.06.2019 04:20 silviamgarcia
The vapor pressure of liquid acetone, ch3coch3, is 100 mm hg at 281 k. a 6.06e-2 g sample of liquid ch3coch3 is placed in a closed, evacuated 360. ml container at a temperature of 281 k. calculate what the ideal gas pressure would be in the container if all of the liquid acetone evaporated.

Answers: 1
Another question on Chemistry

Chemistry, 22.06.2019 04:00
Gymnast always perform on padded mats. how does the mats protect the gymnast
Answers: 2


Chemistry, 22.06.2019 14:30
Aroom with dimensions 7.00m×8.00m×2.50m is to be filled with pure oxygen at 22.0∘c and 1.00 atm. the molar mass of oxygen is 32.0 g/mol. how many moles noxygen of oxygen are required to fill the room? what is the mass moxygen of this oxygen?
Answers: 1

Chemistry, 23.06.2019 00:00
How many moles of water are created if 3 moles of hydrogen react completely with excess oxygen?
Answers: 3
You know the right answer?
The vapor pressure of liquid acetone, ch3coch3, is 100 mm hg at 281 k. a 6.06e-2 g sample of liquid...
Questions









English, 25.01.2022 14:00




Mathematics, 25.01.2022 14:00

Mathematics, 25.01.2022 14:00

Chemistry, 25.01.2022 14:00



English, 25.01.2022 14:00

Mathematics, 25.01.2022 14:00

Mathematics, 25.01.2022 14:00

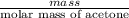
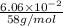
 mole
mole