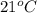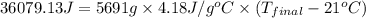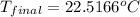Chemistry, 26.06.2019 05:10 kaitlan225
The balanced combustion reaction for c6h6 is 2c6h6(l)+15o2(g)⟶12co2(g)+6h2o(l)+6 542 kj if 8.600 g c6h6 is burned and the heat produced from the burning is added to 5691 g of water at 21 ∘ c, what is the final temperature of the water?

Answers: 2
Another question on Chemistry

Chemistry, 22.06.2019 10:10
What shape would a molecule with two bound groups and two lone pairs have?
Answers: 1

Chemistry, 22.06.2019 16:10
Predict the reactants of this chemical reaction. that is, fill in the left side of the chemical equation. be sure the equation you submit is balanced. (you can edit both sides of the equation to balance it, if you need to.) note: you are writing the molecular, and not the net ionic equation. > cacl2(aq) + h20(l)
Answers: 2

Chemistry, 22.06.2019 21:00
Which answer tells the reason the earth’s climate is getting warmer? too many animals are becoming extinct. large glaciers are melting in antarctica. the earth is moving closer to the sun. driving cars gives off gases that trap heat in the atmosphere.
Answers: 1

Chemistry, 22.06.2019 23:30
Substance a is a nonpolar liquid and has only dispersion forces among its constituent particles. substance b is also a nonpolar liquid and has about the same magnitude of dispersion forces among its constituent particles. when substance a and b are combined, they spontaneously mix.
Answers: 1
You know the right answer?
The balanced combustion reaction for c6h6 is 2c6h6(l)+15o2(g)⟶12co2(g)+6h2o(l)+6 542 kj if 8.600 g c...
Questions




Computers and Technology, 27.04.2021 17:20

Mathematics, 27.04.2021 17:20

Mathematics, 27.04.2021 17:20

Computers and Technology, 27.04.2021 17:20

Chemistry, 27.04.2021 17:20

Mathematics, 27.04.2021 17:20

Mathematics, 27.04.2021 17:20

Mathematics, 27.04.2021 17:20

Mathematics, 27.04.2021 17:20


Mathematics, 27.04.2021 17:20


Chemistry, 27.04.2021 17:20

Mathematics, 27.04.2021 17:20



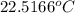
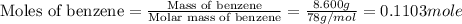
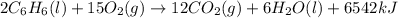
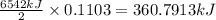 of energy on combustion.
of energy on combustion.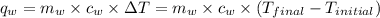
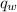 = heat released = 360.7913 kJ = 36079.13 J
= heat released = 360.7913 kJ = 36079.13 J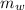 = mass of water = 5691 g
= mass of water = 5691 g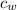 = specific heat of water=
= specific heat of water= 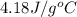
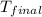 = final temperature = ?
= final temperature = ?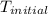 = initial temperature =
= initial temperature =