
Chemistry, 26.06.2019 05:20 sindy35111
The combustion of ethane (c2h6)(c2h6) produces carbon dioxide and steam. 2c2h6(g)+7o2(g)⟶4co2(g)+6h2o(g) 2c2h6(g)+7o2(g)⟶4co2(g)+6h2o(g) how many moles of co2co2 are produced when 5.95 mol5.95 mol of ethane is burned in an excess of oxygen? moles of co2: co2:

Answers: 1
Another question on Chemistry

Chemistry, 22.06.2019 02:30
In pea plants, the allele for tallness (t) is dominant to the allele for shortness (t). in the cross between a tall pea plant and a short pea plant shown below, what is the probability that the resulting offspring will be tall? whats the percent
Answers: 1

Chemistry, 22.06.2019 04:30
Why are people not able to scuba dive in the deep part of the ocean
Answers: 2

Chemistry, 22.06.2019 12:00
Which statement best explains the relationship between an area is geography and the temperature of its surface water
Answers: 1

Chemistry, 22.06.2019 19:00
Imagine that a new planet is discovered with two moons of equal mass: moon a and moon b. the mass of the new planet is greater than the combined mass of its moons. moon a is farther away from the new planet than moon b. what is the planet's gravitational pull on moon a compared to the planet's gravitational pull on moon b? the planet's gravity repels moon a with a greater force than it repels moon b, which is why moon a is farther away. the gravitational pull on moon b is greater than on moon a because moon b is closer to the new planet than moon a. the gravitational pull on moon b is greater than on moon a because moon b is farther away from the new planet than moon a. the gravitational pull on moon a is the same as the gravitational pull on moon b because distance does not affect the planet's gravity.
Answers: 1
You know the right answer?
The combustion of ethane (c2h6)(c2h6) produces carbon dioxide and steam. 2c2h6(g)+7o2(g)⟶4co2(g)+6h2...
Questions

Social Studies, 06.11.2019 07:31

Mathematics, 06.11.2019 07:31

Mathematics, 06.11.2019 07:31

Mathematics, 06.11.2019 07:31





Mathematics, 06.11.2019 07:31




Social Studies, 06.11.2019 07:31



Mathematics, 06.11.2019 07:31


Mathematics, 06.11.2019 07:31

Mathematics, 06.11.2019 07:31

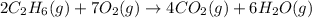
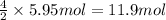 of carbon-dioxide
of carbon-dioxide

