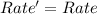Chemistry, 26.06.2019 06:10 katiem7608
Areaction in which a, b, and c react to form products is zero order in a, one-half order in b, and second order in c. by what factor does the reaction rate change if [a] is doubled (and the other reactant concentrations are held constant)?

Answers: 1
Another question on Chemistry

Chemistry, 22.06.2019 09:30
Mike and mitchell decide to have a foot race. they mark off a stretch of 100 yards, and recruit cindy to work the stopwatch. after running the race and looking at the results, cindy declared that mitchell was the fastest. so how did the boys times compare?
Answers: 3

Chemistry, 22.06.2019 13:50
What happens when an atom of sulfur combines with two atoms of chlorine to produce sci2? a. each chlorine atom shares a pair of electrons with the sulfur atom. b. an electron is transferred from each chlorine atom to the sulfur atom. c. an electron is transferred from the sulfur atom to each chlorine atom. d. each chlorine atom shares all its valence electrons with the sulfur atom.
Answers: 2

Chemistry, 22.06.2019 17:00
Reduction is a reaction which results in a in electrons and a in positive charge of the atom or ion 1) a- loss 1) b- gain 2) a-increase 2) b-decrease
Answers: 1

Chemistry, 22.06.2019 18:00
The fact that the total amount of energy in a system remains constant is a(n)
Answers: 1
You know the right answer?
Areaction in which a, b, and c react to form products is zero order in a, one-half order in b, and s...
Questions




Computers and Technology, 17.09.2019 06:30

History, 17.09.2019 06:30


History, 17.09.2019 06:30


Social Studies, 17.09.2019 06:30


Mathematics, 17.09.2019 06:30



Social Studies, 17.09.2019 06:30

History, 17.09.2019 06:30





Mathematics, 17.09.2019 06:30


![Rate=k[A]^x[B]^y[C]^z](/tpl/images/0018/4732/b91f4.png)
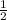
![Rate=k[A]^0[B]^{\frac{1}{2}}[C]^2](/tpl/images/0018/4732/f55a3.png)
![Rate'=k[2A]^0[B]^{\frac{1}{2}}[C]^2](/tpl/images/0018/4732/4c93e.png)
![Rate'=k[2]^0[A]^0[B]^{\frac{1}{2}}[C]^2](/tpl/images/0018/4732/36c04.png)
![Rate'=k[A]^0[B]^{\frac{1}{2}}[C]^2](/tpl/images/0018/4732/23a75.png)