
Chemistry, 26.06.2019 16:10 pchisholm100
Aluminum chloride can be formed from its elements:
(i) 2al(s) + 3cl2 (g) ⟶ 2alcl3 (s) δh° = ? use the reactions here to determine the δh° for reaction (i):
(ii) hcl(g) ⟶ hcl(aq) δh(ii) ° = −74.8 kj
(iii) h2 (g) + cl2 (g) ⟶ 2hcl(g) δh(iii) ° = −185 kj
(iv) alcl3 (aq) ⟶ alcl3 (s) δh(iv) ° = +323 kj/mol
(v) 2al(s) + 6hcl(aq) ⟶ 2alcl3 (aq) + 3h2 (g) δh(v) ° = −1049 kj

Answers: 1
Another question on Chemistry

Chemistry, 22.06.2019 12:40
When 13.3 g koh is dissolved in 102.7 g of water in a coffee-cup calorimeter, the temperature rises from 21.4 °c to 31.53 °c. what is the enthalpy change per gram of koh (j/g) dissolved in the water? * take the density of water as 1.00 g/ml. * assume that the solution has a specific heat capacity of 4.18 j/g*k. enter to 1 decimal place. do not forget the appropriate sign /(+). canvas may auto-delete the (+) sign
Answers: 2

Chemistry, 22.06.2019 14:10
Precision can be defined as the o exact center of a data set. o reproducibility of a measured value. o correlation between two variables that are measured in a data set agreement between a measured value and an accepted value.
Answers: 2

Chemistry, 22.06.2019 19:00
A4.86 g piece of metal was placed in a graduated cylinder containing 15.5 ml of water. the water level rose to 17.3 ml. what is the density of the metal. i need the steps of how to solve it to so i can use a formula to work out other problems.
Answers: 1

Chemistry, 23.06.2019 03:20
High-pressure liquid chromatography (hplc) is a method used in chemistry and biochemistry to purify chemical substances. the pressures used in this procedure range from around 500 kilopascals (500,000 pa) to about 60,000 kpa (60,000,000 pa). it is often convenient to know the pressure in torr. if an hplc procedure is running at a pressure of 1.03×108 pa , what is its running pressure in torr?
Answers: 3
You know the right answer?
Aluminum chloride can be formed from its elements:
(i) 2al(s) + 3cl2 (g) ⟶ 2alcl3 (s) δh° =...
(i) 2al(s) + 3cl2 (g) ⟶ 2alcl3 (s) δh° =...
Questions





Mathematics, 28.10.2020 16:30















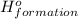 for the reaction is -1406.8 kJ.
for the reaction is -1406.8 kJ.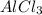 is:
is: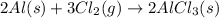
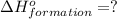
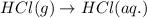
 ( × 6)
( × 6)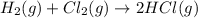
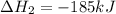 ( × 3)
( × 3)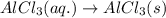
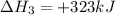 ( × 2)
( × 2)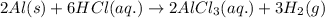
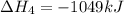
![\Delta H^o_{formation}=[6\times \Delta H_1]+[3\times \Delta H_2]+[2\times \Delta H_3]+[1\times \Delta H_4]](/tpl/images/0020/0754/79ecd.png)
![\Delta H^o_{formation}=[(-74.8\times 6)+(-185\times 3)+(323\times 2)+(-1049\times 1)]=-1406.8kJ](/tpl/images/0020/0754/37adf.png)


