Chemistry, 26.06.2019 17:10 swelch2010
Consider the following reaction at a high temperature. br2(g) ⇆ 2br(g) when 1.35 moles of br2 are put in a 0.780−l flask, 3.60 percent of the br2 undergoes dissociation. calculate the equilibrium constant kc for the reaction.

Answers: 1
Another question on Chemistry

Chemistry, 21.06.2019 21:00
Read these sentences from the text. near the equator, the tropics receive the most rain on a consistent basis. as a result, the fresh water falling into the ocean decrease the salinity of the surface water in that region. [. .] . . as the salt content of sea water increases, so does its density. what can you infer about how rain affects the density of surface water near the equator?
Answers: 1

Chemistry, 22.06.2019 05:00
Use the table to identify the phase and phase changes of the elements under the given conditions. write the name of the substance, phase, or phase change
Answers: 3

Chemistry, 22.06.2019 08:30
For each of the compounds below, show that the charges on the ions add up to zero. a. kbr b. cao c. li(2)o d. cacl(2) e. alcl(3)
Answers: 2

You know the right answer?
Consider the following reaction at a high temperature. br2(g) ⇆ 2br(g) when 1.35 moles of br2 are pu...
Questions

Mathematics, 23.08.2021 23:00







Mathematics, 23.08.2021 23:10

Mathematics, 23.08.2021 23:10


Biology, 23.08.2021 23:10


Mathematics, 23.08.2021 23:10


Social Studies, 23.08.2021 23:10

Mathematics, 23.08.2021 23:10

Physics, 23.08.2021 23:10



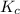 for the reaction is, 0.1133
for the reaction is, 0.1133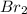 .
.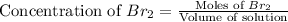
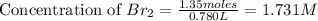
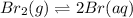
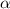 = 1.2 %
= 1.2 %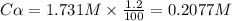
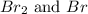 at equilibrium.
at equilibrium.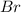 = 2x = 2 × 0.2077 = 0.4154 M
= 2x = 2 × 0.2077 = 0.4154 M![K_c=\frac{[Br]^2}{[Br_2]}](/tpl/images/0020/2368/df6eb.png)