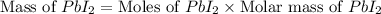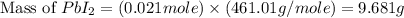The balanced equation for the reaction of aqueous pb(clo3)2 with aqueous nai is pb(clo3)2(aq)+2nai(aq)⟶pbi2(s)+2nac lo3(aq) what mass of precipitate will form if 1.50 l of concentrated pb(clo3)2 is mixed with 0.200 l of 0.210 m nai? assume the reaction goes to completion. mass of precipitate:

Answers: 3
Another question on Chemistry

Chemistry, 21.06.2019 18:30
A6.10 m nacl can be made by adding [x]g of nacl to a container and making the volume of water up to the 1.00 l line
Answers: 1

Chemistry, 22.06.2019 12:00
Why are people not able to skip a dive to the deepest part of the ocean
Answers: 1

Chemistry, 22.06.2019 13:30
Some animals that try to adapt to climate changes eventually die due to starvation, as climate change alters the web.
Answers: 2

Chemistry, 22.06.2019 18:00
How does climate change cause the ocean's thermohaline current to slow down?
Answers: 3
You know the right answer?
The balanced equation for the reaction of aqueous pb(clo3)2 with aqueous nai is pb(clo3)2(aq)+2nai(a...
Questions

Mathematics, 02.02.2021 01:00


Mathematics, 02.02.2021 01:00

Chemistry, 02.02.2021 01:00

Mathematics, 02.02.2021 01:00

Mathematics, 02.02.2021 01:00




Biology, 02.02.2021 01:00

Mathematics, 02.02.2021 01:00

Mathematics, 02.02.2021 01:00

Mathematics, 02.02.2021 01:00


Mathematics, 02.02.2021 01:00


English, 02.02.2021 01:00

Mathematics, 02.02.2021 01:00

Social Studies, 02.02.2021 01:00

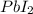 precipitate produced will be, 9.681 grams.
precipitate produced will be, 9.681 grams. .
.
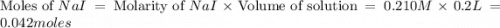
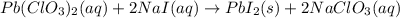
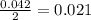 moles of
moles of