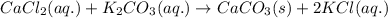Chemistry, 28.01.2020 14:45 glocurlsprinces
Consider the reaction. cacl2(aq)+k2co3(aq)⟶caco3+2kcl. identify the precipitate, or lack thereof, for the reaction. (a) kcl (b) caco3 (c) no precipitate

Answers: 2
Another question on Chemistry

Chemistry, 21.06.2019 22:40
How many electrons does silver have to give up in order to achieve a sido noble gas electron configuration
Answers: 1


Chemistry, 23.06.2019 01:30
Astudent states that 9.0 g of baking soda will form an unsaturated solution in 100 g of water. what do you need to know to decide whether this statement is correct? a. the temperature of the water and the molar mass of baking soda b. the percent by volume of the solution and the solubility of baking soda c. the temperature of the water and the solubility of baking soda at that temperature
Answers: 1

Chemistry, 23.06.2019 03:00
Asample of sea water contains 6.28g of sodium chloride per litre of solution. how many milligrams of sodium chloride would be contained in 15.0ml of this solution?
Answers: 3
You know the right answer?
Consider the reaction. cacl2(aq)+k2co3(aq)⟶caco3+2kcl. identify the precipitate, or lack thereof, fo...
Questions


Mathematics, 01.02.2020 05:45

Social Studies, 01.02.2020 05:45







History, 01.02.2020 05:45

Mathematics, 01.02.2020 05:45




Mathematics, 01.02.2020 05:45

History, 01.02.2020 05:45

Mathematics, 01.02.2020 05:45

History, 01.02.2020 05:45

Health, 01.02.2020 05:45