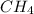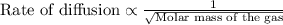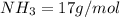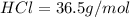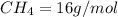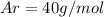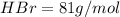Chemistry, 27.06.2019 02:10 kcceff4085
Of the following gases, will have the greatest rate of effusion at a given temperature. of the following gases, will have the greatest rate of effusion at a given temperature. nh3 hcl ch4 ar hbr

Answers: 3
Another question on Chemistry

Chemistry, 21.06.2019 19:30
The ph of carrots are 5.0 how it is classified a.acidic b.basic c.indicator d.neutral
Answers: 2

Chemistry, 22.06.2019 12:00
Explain what happens at the saturation point when adding salt to water at room temperature.
Answers: 1

Chemistry, 22.06.2019 17:10
Calculate the estimated density of each ball. use the formula d = m/v where d is the density, m is the mass, and v is the volume. record your calculations in table a of your student guide. given that the density of water is 1.0 g/cm3, make a prediction about whether each ball will float in water. record your prediction in table a. what is the estimated density of the table tennis ball? record your answer to the nearest hundredth
Answers: 2

Chemistry, 22.06.2019 20:00
What happens to the temperature of a substance when the average kinetic energy of its particles increases?
Answers: 3
You know the right answer?
Of the following gases, will have the greatest rate of effusion at a given temperature. of the foll...
Questions

Mathematics, 25.03.2021 01:00

Mathematics, 25.03.2021 01:00

Mathematics, 25.03.2021 01:00



English, 25.03.2021 01:00




Social Studies, 25.03.2021 01:00

Mathematics, 25.03.2021 01:00

Mathematics, 25.03.2021 01:00


Mathematics, 25.03.2021 01:00

Mathematics, 25.03.2021 01:00

Health, 25.03.2021 01:00



English, 25.03.2021 01:00

Mathematics, 25.03.2021 01:00