
Chemistry, 27.06.2019 03:30 NatalieAllen11
Magnesium and nitrogen react in a combination reaction to produce magnesium nitride: 3 mg + n2→ mg3n2 in a particular experiment, a 5.65-g sample of n2 reacts completely. the mass of mg consumed is g.

Answers: 2
Another question on Chemistry

Chemistry, 22.06.2019 07:00
Which atom or ion is the largest? a. k b. k+ c. ca d. ca2+ e. li
Answers: 1

Chemistry, 22.06.2019 08:00
Me i dont know what to do! the table compares the number of electrons in two unknown neutral atoms. comparison of electrons atom number of electrons a 10 d 11 use this information to determine the number of valence electrons in the atoms. which of the following correctly compares the stability of the two atoms? both are unreactive. both are highly reactive. a is unreactive and d is reactive. a is reactive and d is unreactive.
Answers: 1

Chemistry, 22.06.2019 14:00
8.98 dm3 of hydrogen gas is collected at 38.8 °c. find the volume the gas will occupy at -39.9 °c if the pressure remains constant.
Answers: 3

Chemistry, 22.06.2019 14:30
Calculate the mass of carbon in 97.0 g of sucrose c12h22o11
Answers: 3
You know the right answer?
Magnesium and nitrogen react in a combination reaction to produce magnesium nitride: 3 mg + n2→ mg3...
Questions


History, 06.05.2020 01:16

Mathematics, 06.05.2020 01:16

English, 06.05.2020 01:16

History, 06.05.2020 01:16


Mathematics, 06.05.2020 01:16





Mathematics, 06.05.2020 01:16

Mathematics, 06.05.2020 01:16

Chemistry, 06.05.2020 01:16

History, 06.05.2020 01:16

Chemistry, 06.05.2020 01:16


Mathematics, 06.05.2020 01:16

Chemistry, 06.05.2020 01:16

 .....(1)
.....(1)
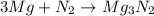
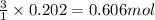 of magnesium.
of magnesium.


