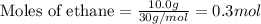Chemistry, 27.06.2019 10:10 Chatoloko231
Consider a sample of 10.0 g of the gaseous hydrocarbon c2h6 to answer the following question: how many moles are present in this sample?
when answering the question, include the following:
state how to find the molar mass for the hydrocarbon.
state how you know if you need to multiply or divide by the molar mass.
give the correct number of significant figures and explain why the answer has that many significant figures.

Answers: 3
Another question on Chemistry

Chemistry, 22.06.2019 00:00
Substance x has a fixed volume, and the attraction between its particles is strong .substance y had widely spread out particles and can be compressed what can most likely be concluded about these substances
Answers: 2

Chemistry, 22.06.2019 04:30
Why are people not able to scuba dive in the deep part of the ocean
Answers: 2

Chemistry, 22.06.2019 05:30
Modern weaponry has increased the number of deaths in wars and violent conflicts.
Answers: 3

You know the right answer?
Consider a sample of 10.0 g of the gaseous hydrocarbon c2h6 to answer the following question: how m...
Questions

Mathematics, 02.02.2021 03:40


Mathematics, 02.02.2021 03:40

Chemistry, 02.02.2021 03:40

Mathematics, 02.02.2021 03:40



Social Studies, 02.02.2021 03:40

Mathematics, 02.02.2021 03:40

Social Studies, 02.02.2021 03:40

Mathematics, 02.02.2021 03:40


Chemistry, 02.02.2021 03:40

Mathematics, 02.02.2021 03:40

Mathematics, 02.02.2021 03:40

Mathematics, 02.02.2021 03:40

Computers and Technology, 02.02.2021 03:40


Mathematics, 02.02.2021 03:40

Computers and Technology, 02.02.2021 03:40

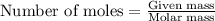
![[(2\times 12)+(6\times 1)]=30g/mol](/tpl/images/0022/9571/19171.png)