

Answers: 1
Another question on Chemistry

Chemistry, 22.06.2019 15:30
A1.5l container holds p.50 grams of an unknown gas at a pressure of 0.44 atm and a temperature of 50.c what is the molar mass of the unknown gas
Answers: 1

Chemistry, 22.06.2019 17:10
Increasing the substrate concentration in an enzymatic reaction could overcome which of the following? a) the need for a coenzymeb) allosteric inhibitionc) competitive inhibitiond) insufficient cofactors
Answers: 1

Chemistry, 22.06.2019 20:00
Many free radicals combine to form molecules that do not contain any unpaired electrons. the driving force for the radical–radical combination reaction is the formation of a new electron‑pair bond. consider the chemical equation. n(g)+no(g)⟶nno(g) n(g)+no(g)⟶nno(g) write lewis formulas for the reactant and product species in the chemical equation. include nonbonding electrons. n(g)n(g) select draw rings more erase select draw rings more erase select draw rings more erase n no(g)
Answers: 1

Chemistry, 22.06.2019 22:30
Draw the aromatic compound toluene (methylbenzene). show all hydrogen atoms, including those on the ring.
Answers: 1
You know the right answer?
Given the following equation: 4 nh3 (g)5 o2 (g) > 4 no (g) + 6 h20 (i) how many moles of nh3 is...
Questions

Physics, 20.09.2020 05:01

English, 20.09.2020 05:01

English, 20.09.2020 05:01

English, 20.09.2020 05:01

Mathematics, 20.09.2020 05:01




Mathematics, 20.09.2020 05:01


Mathematics, 20.09.2020 05:01




Biology, 20.09.2020 05:01

Mathematics, 20.09.2020 05:01


Mathematics, 20.09.2020 05:01


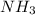 is required to react with 25.7 grams of
is required to react with 25.7 grams of 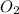 .
.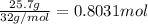
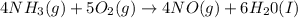
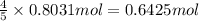 of ammonia gas
of ammonia gas

