

Answers: 1
Another question on Chemistry


Chemistry, 22.06.2019 05:30
Choose all the answers that apply. as ocean depth increases, temperature decreases temperature increases pressure increases pressure decreases salinity increases density increases
Answers: 2


Chemistry, 22.06.2019 17:00
Which statement is true about a catalyst? a: a catalyst decreases the rate of the reaction. b. a catalyst is consumed during a chemical reaction. c. a catalyst lowers the activation energy of a reaction. d. a catalyst increases the reactant concentration during a reaction.
Answers: 1
You know the right answer?
Given the following equation: 4 nh3 (g)5 o2 (g) > 4 no (g) 6 h20 () + how many moles of h20 is...
Questions

Mathematics, 05.09.2019 05:30

Mathematics, 05.09.2019 05:30


History, 05.09.2019 05:30


History, 05.09.2019 05:30



English, 05.09.2019 05:30


Mathematics, 05.09.2019 05:30

History, 05.09.2019 05:30

Physics, 05.09.2019 05:30


Mathematics, 05.09.2019 05:30

Mathematics, 05.09.2019 05:30

Mathematics, 05.09.2019 05:30



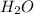 produced are, 0.66 mole.
produced are, 0.66 mole.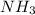 = 0.44 mole
= 0.44 mole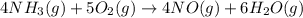
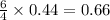 moles of
moles of 


