Chemistry, 27.06.2019 19:30 jeffmacdonald1976
Given the following equation: |4 nh3 (g) + 5 о2 (g) —> 4 no (g) + 6 h20 () how many grams of h20 is produced if 21.1 grams of nh3 reacts with 73.9 grams of o2?

Answers: 3
Another question on Chemistry

Chemistry, 21.06.2019 20:30
The balanced chemical equation for this lab is: 3cucl2(aq) + 2al(s) 3cu(s) + 2alcl3(aq) if 10.5 g copper chloride react with 12.4 g aluminum, what is the limiting reactant?
Answers: 3

Chemistry, 21.06.2019 21:30
Which statements are true about electrolysis? check all that apply. electrolysis requires an acid be present. electrolysis is described by two half-reactions. electrolysis is not an industrial process. electrolysis results in commercially valuable products. electrolysis involves the transfer of electrons. reduction results in the loss of electrons. oxidation results in the loss of electrons.
Answers: 1

Chemistry, 22.06.2019 02:30
98 ! and brainliest plz ! the below reaction can be categorized as more than one type of reaction. which reactions are these, and what are the types of reactions?
Answers: 1

Chemistry, 22.06.2019 06:30
Particle model to predict what will happen if a sharp object creates a hole in the soccer ball
Answers: 2
You know the right answer?
Given the following equation: |4 nh3 (g) + 5 о2 (g) —> 4 no (g) + 6 h20 () how many grams of h2...
Questions









Spanish, 12.11.2020 16:30

Mathematics, 12.11.2020 16:30

Mathematics, 12.11.2020 16:30









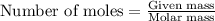 ....(1)
....(1)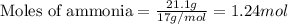
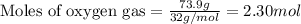
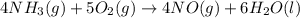
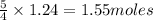 of oxygen gas.
of oxygen gas.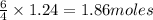 of water.
of water.