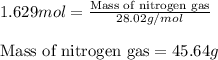Chemistry, 27.06.2019 19:30 38saferguson
Determine the limiting reactant (lr) and the mass (in g) of nitrogen that can be formed from 50.0 g n204 and 45.0 g n2h4. some possibly useful molar masses are as follows: n2o4 92.02 g/mol, n2h4 32.05 g/mol n204) 2 n2h4(1)3 n2(g) + 4 h2o(g)

Answers: 2
Another question on Chemistry


Chemistry, 22.06.2019 07:20
After watching the video "zinc strip in copper nitrate solution", and reading the instructions, click on the link labeled "start" just below the drawing of the pencil tip. follow the direction to complete the 3x3 grid. answer the below questions for the portion of the activity in which sn(s) is placed in agno3(aq)
Answers: 1


Chemistry, 22.06.2019 14:30
In water, a strong acid will break down into its component parts. a. completely b. partly c. never in water, a weak base will break down into its component parts. a. completely b. partly c. never
Answers: 2
You know the right answer?
Determine the limiting reactant (lr) and the mass (in g) of nitrogen that can be formed from 50.0 g...
Questions


Mathematics, 23.11.2019 04:31

Mathematics, 23.11.2019 04:31

Biology, 23.11.2019 04:31



Mathematics, 23.11.2019 04:31

Computers and Technology, 23.11.2019 04:31

Mathematics, 23.11.2019 04:31



History, 23.11.2019 04:31

Chemistry, 23.11.2019 04:31

English, 23.11.2019 04:31

Mathematics, 23.11.2019 04:31

History, 23.11.2019 04:31


Mathematics, 23.11.2019 04:31

Social Studies, 23.11.2019 04:31

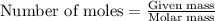 .....(1)
.....(1)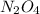
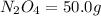
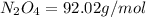
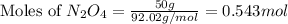
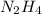
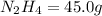
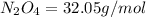
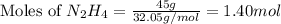
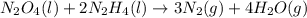
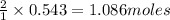 of
of 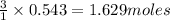 of nitrogen gas.
of nitrogen gas.